|
January 2022

Federal No Surprises Act prohibits ‘surprise billing’
What you need to know
Blue Cross and BCN align with the federal No Surprises Act, which prohibits nonparticipating professional providers from surprise billing for emergency services, some non-emergency services and air ambulance services.
Blue Cross Blue Shield of Michigan and Blue Care Network have made changes to align with the federal No Surprises Act, effective Jan. 1, 2022. The law, part of the Consolidated Appropriations Act, or CAA, prohibits surprise billing nationwide for emergency, some non-emergency and air ambulance services.
Surprise billing is when a member unknowingly receives care from a health care provider who doesn’t participate with the member’s health insurance plan. The member then receives an unexpected bill for the difference between the health plan’s payment and what the health care provider charges.
Blue Cross and BCN already align with the state surprise billing law, which went into effect Oct. 22, 2020. The state law prohibits surprise billing by Michigan nonparticipating professional providers for emergency services and some non-emergency services.
As of Jan, 1, 2022, Blue Cross is handling claims according to the federal law for self-funded ERISA plans, grandfathered plans and federal health plans. We’re also following the federal law for fully insured plans and self-funded state or local government plans. However, these plans will still follow the state law for professional provider payment rates and arbitration procedures.
Blue Cross maintains the broadest network of providers in Michigan, and helps ensure access to high-quality, in-network care across the country through our relationship with the Blue Cross Blue Shield Association.
If you have questions, contact Provider Inquiry at the appropriate number below:
- Blue Cross Blue Shield of Michigan
- Michigan physicians and other professional providers of care: 1-800-344-8525
- Providers outside of Michigan: 1-800-676-2583
- Michigan hospital and facility providers: 1-800-249-5103
- Hospital and facility providers outside of Michigan: 1-800-676-2583
- Blue Care Network
- Professional providers: 1-800-344-8525
- Ancillary and facility providers:1-800-249-5103
Our self‑service tools can help you find answers to your questions
We encourage our provider community to use our self-service tools to avoid long wait times to reach a representative.
Blue Cross Blue Shield of Michigan is committed to helping you become more knowledgeable about such topics as claims, benefits, authorizations, referrals and medical policy.
When you contact Provider Inquiry, you have an opportunity to interact with our automated system before speaking with a service representative. We urge you to take advantage of that useful option.
Additional self-service tools include web-DENIS, Provider Secured Services, online provider manuals and ereferrals.bcbsm.com.
Here’s an overview of key resources:
Need contact information?
Eligibility and benefits:
Eligibility precertification and preauthorization contacts
Claims:
Claims submission and status contacts
Main contact page:
Contact Us
Have a question about authorizations and referrals?
Visit ereferrals.bcbsm.com before contacting Provider Inquiry. Select the Blue Cross or BCN tab. You’re likely to find your questions answered at this comprehensive site.
Have a medical policy question?
Check out our online provider manuals in one of two ways:
- After logging in as a provider at bcbsm.com, click on Provider Manuals in the lower right section of the page, or
- From the homepage of web-DENIS, click on Provider Manuals at the left.
Other resources
Get ready for Availity: Learn more about alerts and provider resources
 This is part of a series of articles on our move to Availity® for our provider portal. This is part of a series of articles on our move to Availity® for our provider portal.
After we transition to the Availity provider portal, you’ll still be able to access important alerts, resources and publications for Blue Cross Blue Shield of Michigan and Blue Care Network. However, how you find this information will change, and you’ll also see a new and improved look.
Currently, you read broadcast messages when you click on web-DENIS. They’re listed in the center of the screen under Welcome to web-DENIS. In the left navigation of web-DENIS, you can click on one of the following to find the information you need to do business with us:
- BCBSM Provider Publications and Resources
- BCN Provider Publications and Resources
- Provider Manuals
New way to find alerts and provider resources
After the transition to Availity, what you see now as web-DENIS broadcast messages will simply be called “alerts.” You’ll find them, along with provider publications, resources and manuals, within the Blue Cross and BCN payer space. After logging in to Availity, you’ll click on Payer Spaces on the top navigation bar, then click on BCBSM and BCN.
This will bring you to our payer space, which will include information for both Blue Cross and BCN. You’ll see three tabs:
- Applications — Our payer space will always open on the Applications tab. This is where you’ll find applications specific to Blue Cross and BCN. For example, you’ll find e-referral, Benefit Explainer, Health e-Blue℠, and Provider Enrollment and Change Self-Service.
- Resources — Clicking on the Resources tab will show you links to our websites and provider manuals where you can find important information you need.
- News and Announcements — This tab will connect you with our provider alerts, so you can find breaking or critical news you need to know.
Improvements
We’re working to make it easier for you to find what you need. For example, our new Provider Resources website will have information for both Blue Cross and BCN within a single site and will include a new search feature. You can access the website through the payer space Resources tab.
Also, within the payer space Applications and Resources tabs, you’ll be able to select specific items that you use frequently as “favorites.” They’ll be accessible from your Availity top navigation bar no matter where you are in the portal.
Questions?
If you have questions about the move to Availity, please check our Frequently Asked Questions document first. If your question isn’t already answered there, submit your question to ProviderPortalQuestions@bcbsm.com, so we can consider adding it to the FAQ document.
Previous articles about Availity
We’re providing a series of articles focusing on our move to Availity for our provider portal. Here are the articles we’ve already published in case you missed them:
Availity is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services.
2022 HCPCS and CPT Update now available
Each year, we publish our HCPCS and CPT Update document, containing new and deleted HCPCS and CPT codes, and post it on web-DENIS.
To access the 2022 document, follow these steps:
- From the homepage of web-DENIS, click on BCBSM Provider Publications and Resources.
- Click on Newsletters and Resources.
- Click on Clinical Criteria & Resources.
- Scroll down to the HCPCS and CPT Updates section of the page.
You’ll want to use it as a reference guide for new CPT and HCPCS codes for 2022. As future updates occur throughout the year, we’ll publish Record articles that outline the changes.
Billing chart: Blue Cross highlights medical, benefit policy changes
You’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart.
This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures.
You will also see that descriptions for the codes are no longer included. This is a result of recent negotiations with the AMA on use of the codes.
We will publish information about new BCBS groups or changes to group benefits under the Group Benefit Changes heading.
For more detailed descriptions of the BCBSM policies for these procedures, please check under the Medical/Payment Policy tab in Explainer on web-DENIS. To access this online information:
- Log in to web-DENIS.
- Click on BCBSM Provider Publications & Resources.
- Click on Benefit Policy for a Code.
- Click on Topic.
- Under Topic Criteria, click on the drop-down arrow next to Choose Identifier Type and then click on HCPCS Code.
- Enter the procedure code.
- Click on Finish.
- Click on Search.
| Code* |
BCBSM changes to:
Basic Benefit and Medical Policy, Group
Variations Payment Policy, Guidelines
|
| NEW PAYABLE PROCEDURES |
31647, 31648, 31649, 31651 |
Basic benefit and medical policy
Bronchial valves
The insertion of endobronchial valves is established in adult patients with respiratory compromise from hyperinflation associated with severe heterogenous lung emphysema with little to no collateral ventilation.
The insertion of endobronchial valves is established for persistent bronchopleural air leak causing pneumothorax that isn’t improving five or more days after chest tube insertion.
This policy is effective Sept. 1, 2021.
Payment policy
Inclusions:
Criteria for pleural air leak
Bronchopleural air leak not improving five or more days after a chest tube placement, when site of the air leak can be identified by balloon occlusion of the distal affected bronchus.
Criteria for emphysema
Respiratory insufficiency caused by bullous emphysema in a patient found after a multidisciplinary evaluation not to be a candidate for lung volume reduction surgery.
Inclusions:
- Ex-smokers
- PFT:
- Post BD FEV1 15 – 45%
- TLC ≥100%
- RV ≥175%
- ABG with pCO2 <60
- Completed pulmonary rehabilitation program or enrollment in a pulmonary rehabilitation program of at least six to eight sessions or attestation from physician that the patient has received adequate pulmonary rehabilitation to proceed with surgery
- CT imaging confirming intact fissure between lobes
Exclusions:
Any general contraindications to bronchoscopy or general anesthesia:
- Lung findings:
- Pulmonary nodule requiring workup
- Giant bullae (>1/3 hemithorax)
- CV event in prior six months (needs TTE within three months)
- Recent CVA (three months)
- Known uncontrolled PAH without PAP >45
|
80145, 80230
Experimental:
80280, 84999**
**Used to report not otherwise classified service |
Basic benefit and medical policy
Measurement of serum and anti-drug antibody levels for selected biologic agents
Measurement of biologic agent drug levels and, if low, anti-drug antibody levels in children with inflammatory bowel disease, or IBD, is established, effective June 1, 2021.
Measurement of antidrug antibodies in other patients being treated with a biologic agent, either alone or as a combination test, which includes the measurement of serum TNF blocking agent levels, is considered experimental. The use of these tests hasn’t been clinically proven to improve patient clinical outcomes or alter patient management.
Payment policy:
Modifiers 26 and TC don’t apply to these codes. Not payable when performed in an office location. Payable for diagnoses related to inflammatory bowel disease only.
Inclusions:
Biologic agent drug levels are established in children (under the age of 21) who are:
- Diagnosed with inflammatory bowel disease and
- Being treated with either Adalimumab or Infliximab
- Being monitored for their response to the agent by biologic agent drug level
If the biologic agent drug level is below the therapeutic range, anti-drug antibody level is established.
Exclusions:
- Individuals age 21 and older
- Any condition other than inflammatory bowel disease
|
81425, 81426, 81427 |
Basic benefit and medical policy
Whole genome sequencing
Rapid whole genome (or exome) sequencing, with trio testing when possible, may be considered established for the evaluation of critically ill infants and children in neonatal or pediatric intensive care with a suspected genetic disorder of unknown etiology when at least one of the following criteria is met:
- Multiple congenital anomalies
- An abnormal laboratory test or clinical features suggests a genetic disease or complex metabolic phenotype
- An abnormal response to standard therapy for a major underlying condition
This policy update is effective March 1, 2021.
Payment policy:
Modifiers 26 and TC aren’t applicable. Payable in an inpatient and outpatient location only.
Exclusions:
Whole genome (and exome) sequencing, WGS or WES, is considered experimental for the diagnosis or screening of genetic disorders.
Note: See medical policy for additional information on WES sequencing.
|
| UPDATES TO PAYABLE PROCEDURES |
J9299 |
Basic benefit and medical policy
Opdivo (nivolumab)
Opdivo (nivolumab), procedure code J9299, is covered for the following updated FDA-approved indications:
- Gastric cancer
- Gastroesophageal junction cancer
- Esophageal adenocarcinoma
For patients with advanced or metastatic gastric cancer, gastroesophageal junction cancer and esophageal adenocarcinoma in combination with fluoropyridine and platinum-containing chemotherapy
Esophageal cancer:
- For patients with completely resected esophageal or gastroesophageal junction cancer with residual pathologic disease, who have received neoadjuvant chemoradiotherapy, or CRT
Note: Opdivo is no longer FDA-approved for the treatment of small cell lung cancer, effective Dec. 29, 2020. |
| POLICY CLARIFICATIONS |
52441, 52442
C9739, C9740 |
Basic benefit and medical policy
Alternative billing guidelines for cystourethroscopy procedure codes *52441 and *52442
Discontinue using cystourethroscopy procedure codes *52441 and *52442. These codes are being discontinued and replaced with Level II HCPCS procedure codes C9739 and C9740 to accommodate the appropriate reimbursement for multiple quantities.
Facilities should report C9739 when performing a single implant and C9740 for each additional permanent adjustable implant along with the applicable quantity.
The effective date of this change is retroactive to Jan. 1, 2020, to accommodate the resubmission of claims where the appropriate reimbursement was affected by multiple quantities. Facilities may resubmit claims processed for dates of service Jan. 1, 2020, through the present. |
81412, 81443, 81479, various**
**If CPT Tier 1 or Tier 2 molecular pathology codes are available for the specific test, they should be used. If the test hasn’t been codified by CPT, the unlisted molecular pathology code *81479 would be used. |
Basic benefit and medical policy
Carrier screening for genetic diseases
The medical policy statement and inclusionary and exclusionary criteria have been updated, effecttive Nov. 1, 2021
Medical policy statement:
Panethnic expanded carrier testing for autosomal recessive and x-linked genetic disorders has been established. It may be considered a useful diagnostic option when indicated.
The safety and effectiveness of targeted carrier testing for autosomal recessive and x-linked genetic disorders have been established. It may be considered a useful diagnostic option when indicated.
Inclusions:
Panethnic expanded carrier testing for autosomal recessive and x-linked genetic disorders when the female is pregnant or is considering pregnancy.a
aIf the initial testing is positive in one partner, then sequential testing in the other partner should be focused on specific gene abnormalities.
Targeted carrier screening for autosomal recessive and x-linked genetic disorders when all the following apply:
- The couple is pregnant or is considering pregnancy.
- The natural history of the disease is well understood and there is a reasonable likelihood that the disease is one with high morbidity in the homozygous or compound heterozygous state.
- Alternative biochemical or other clinical tests to definitively diagnose carrier status aren’t available, or, if available, provide an indeterminate result or are individually less efficacious than genetic testing.
- The genetic test has adequate sensitivity and specificity to guide clinical decision-making and residual risk is understood.
- An association of the marker with the disorder has been established.
Exclusions:
- All targeted and carrier screening panels not meeting the above criteria
- Carrier screen testing of the male partner when the female partner was found not to have risk (i.e., sequential testing)
- Carrier screen testing of the male partner at the same time that the female partner is undergoing carrier screen testing (i.e., simultaneous testing)
|
90587 |
Basic benefit and medical policy
Dengue vaccine
Dengue vaccine, quadrivalent, live, 3-dose schedule, for subcutaneous use is established in individuals ages 9 through 16 years with laboratory-confirmed previous dengue infection who are living in endemic areas.
This is payable for all groups as of its FDA effective date, May 1, 2019. |
90867, 90868, 90869 |
Basic benefit and medical policy
Transcranial magnetic stimulation as treatment for depression
Transcranial magnetic stimulation of the brain has been established. It may be a useful treatment option in specified situations.
Exclusionary criteria have been updated, effective Nov. 1, 2021.
Inclusionary and exclusionary guidelines
Inclusions:
Transcranial magnetic stimulation must be administered by an approved U.S. Food & Drug Administration-cleared device for the treatment of major depressive disorder, or MDD, according to specified stimulation parameters, five days a week for six weeks (total of 30 sessions), followed by a three-week taper of three TMS treatments in one week, two TMS treatments the next week and one TMS treatment in the last week.
Must meet all:
- The member is 18 to 70 years old.
- A drug screen is obtained if indicated by history, current clinical evaluation or a high degree of clinical suspicion.
- A confirmed diagnosis of severe major depressive disorder (single or recurrent episode) measured by evidence-based scales such as Beck Depression Inventory (score 30-63), Zung Self-Rating Depression Scale (>70), PHQ-9 (>20), Hamilton Depression Rating Scale (>20) or Montgomery-Asberg Depression Rating Scale (score >34).
- At least one of the following:
- Current depressive episode treatment:
- Medication treatment resistance, evidenced by:
- Lack of a clinically significant response to 4 trials of psychopharmacologic agents:
- Two single agent trials of antidepressants from at least two different agent classes
- Two augmentation trials with different classes of augmenting agents utilizing either (or both) of the agents used in the single agent trials
Note: Each agent in the treatment trial must have been administered at an adequate course of mono- or poly-drug therapy.
Note: Trial criteria is six weeks of maximal FDA-recommended dosing or maximal tolerated dose of medication with objectively measured evaluation at initiation and during the trial showing no evidence of response (i.e., < 50% reduction of symptoms or scale improvement).
- The patient is unable to tolerate a therapeutic dose of medications. Intolerance is defined as severe somatic or psychological symptoms that can’t be modulated by any means including but not limited to additional medications to ameliorate side effects. Examples of somatic side effects: persistent electrolyte imbalance, pancytopenia, severe weight loss, poorly controlled metabolic syndrome or diabetes. Examples of psychological side effects: suicidal-homicidal thinking/attempts, impulse dyscontrol. Note: A trial of less than one week of a medication is not considered a qualifying trial to establish intolerance.
- The patient has a history of response to rTMS in a previous depressive episode (and it’s been at least three months since the prior episode)
- The patient is a candidate for electroconvulsive therapy; further, electroconvulsive therapy would not be clinically superior to transcranial magnetic stimulation (e.g., in cases with psychosis, acute suicidal risk, catatonia or life-threatening inanition rTMS should not be utilized).
- The patient failed a trial of an evidence-based psychotherapy known to be effective in the treatment of MDD of an adequate frequency and duration without significant improvement in depressive symptoms as documented by standardized rating scales that reliably measure depressive symptoms (e.g., Becks Depression Inventory, Zung Self-Rating Depression Scale, PHQ-9, Hamilton Depression Rating Scale or MADRS).
- Conditions that must be met during the entire rTMS treatment:
- A board-certified psychiatrist, trained in this therapy, must deliver the treatment
- An attendant trained in BCLS, the management of complications (such as seizures), and the use of the equipment must be present
- Adequate resuscitation equipment must be available (e.g., suction and oxygen)
- The facility must maintain awareness of response times of emergency services (either fire/ambulance or “code team”), which should be available within five minutes. These relationships are reviewed on at least a one year basis and include mock drills.
Exclusions:
- All other behavioral health, neuropsychiatric or medical conditions (e.g., anxiety disorders, mood disorders, schizophrenia, Alzheimer’s, dysphagia, seizures, obsessive-compulsive disorder**)
**Individual consideration may be extended to patient’s with OCD based on review of applicable medical records.
- Pregnancy
- Maintenance treatment
- Presence of psychosis in the current episode
- Seizure disorder or any history of seizure, except those induced by ECT or isolated febrile seizures in infancy without subsequent treatment or recurrence
- Presence of an implanted magnetic-sensitive medical device located less than or equal to 30 centimeters from the TMS magnetic coil or other implanted metal items, including but not limited to a cochlear implant, implanted cardioverter defibrillator, pacemaker, vagus nerve stimulator, or metal aneurysm clips or coils, staples, or stents
Note: Dental amalgam fillings aren’t affected by the magnetic field and are acceptable for use with TMS.
- If the patient (or, when indicated, the legal guardian) is unable to understand the risk and benefits of rTMS and provide informed consent
- Presence of a medical or co-morbid psychiatric contraindication to rTMS
- Patient lacks a suitable environmental, or social or professional support system for post-treatment recovery
- There isn’t a reasonable expectation that the patient will be able to adhere to post-procedure recommendations
Note: Caution should be exercised in any situation where the patient’s seizure threshold may be decreased. Examples include:
- Presence in the bloodstream of a variety of agents including, but not limited to, tricyclic antidepressants, clozapine, antivirals, theophylline, amphetamines, PCP, MDMA, alcohol and cocaine as these present a significant risk
- Presence of the following agents including, but not limited to, SSRIs, SNRIs, bupropion, some antipsychotics, chloroquine, some antibiotics and some chemotherapeutic agents as they present a relative risk and should be considered when making risk-benefit assessments
- Withdrawal from alcohol, benzodiazepines, barbiturates and chloral hydrate also present a strong relative hazard
|
Established:
91110
Not covered:
91111, 0355T, 91299, 0651T |
Basic benefit and medical policy
Wireless capsule endoscopy
The medical policy statement and exclusionary criteria have been updated, effecttive Nov. 1, 2021.
Medical policy statement:
Wireless capsule endoscopy has been proven to be safe and effective. It’s a useful therapeutic option for patients meeting patient selection criteria.
The peer-reviewed medical literature is insufficient to determine the effectiveness of the patency capsule (e.g., Given AGILE™). Therefore, it’s considered experimental, including in the evaluation of the patency of the GI tract before wireless capsule endoscopy.
The use of a magnetically controlled wireless capsule (e.g., NaviCam®) is considered experimental. The medical literature is insufficient to determine that this technology results in improved clinical outcomes over standard care.
Inclusionary and exclusionary guidelines:
Inclusions:
- Initial diagnosis in patients with suspected Crohn’s disease without evidence of disease on conventional diagnostic tests such as CT enterography, MR enterography, small-bowel follow-through, or SBFT, and upper and lower endoscopy
- In patients with an established diagnosis of Crohn’s disease, when there are unexpected changes in the course of disease or response to treatment, suggesting the initial diagnosis may be incorrect and re-examination may be indicated.
- Evaluation for the extent of involvement or management of known Crohn’s disease
- Suspected small bowel bleeding, as evidenced by prior inconclusive upper and lower gastrointestinal endoscopic studies
- For surveillance of the small bowel in patients with hereditary GI polyposis syndromes, including familial adenomatous polyposis and Peutz-Jeghers syndrome
Exclusions:
- Evaluation for the extent of involvement or management of known ulcerative colitis
- Evaluation of the esophagus, in patients with gastroesophageal reflux, or GERD, or other esophageal pathologies
- Evaluation of other gastrointestinal diseases not presenting with GI bleeding including, but not limited to, celiac sprue, irritable bowel syndrome, Lynch syndrome, portal hypertensive enteropathy, small bowel neoplasm and unexplained chronic abdominal pain
- Evaluation of the colon including, but not limited to, detection of colonic polyps or colon cancer
- Initial evaluation of patients with acute upper GI bleeding
- Evaluation of patients with evidence of lower GI bleeding who have major risks for colonoscopy or moderate sedation
- Evaluation of patients following incomplete colonoscopy
- Use of wireless capsule for routine colorectal cancer screening, confirmation of lesions or pathology normally within the reach of upper or lower endoscopes
- In patients with known or suspected gastrointestinal obstruction, strictures or fistulas
The patency capsule (e.g., Given AGILE™) is considered experimental, including in the evaluation of the patency of the GI tract before wireless capsule endoscopy.
Use of a magnetically-controlled wireless capsule (e.g., NaviCam®) is considered experimental. |
Established:
93895, 93998 |
Basic benefit and medical policy
U.S. measurement of carotid intima-media as assessment for atherosclerosis
The medical policy statement has been updated, effective Nov. 1, 2021.
Medical policy statement:
Ultrasonographic measurement of carotid artery intima-media thickness to screen, diagnose or manage subclinical atherosclerosis is considered experimental. Although it may be safe, its usefulness hasn’t been definitively proven. |
97545
97546 |
Basic benefit and medical policy
Codes *97545 and *97546 will reject
Effective Nov. 1, 2021, procedure codes *97545 and *97546 will reject as not a benefit, subscriber liable. |
C9399
J3490
J3590 |
Basic benefit and medical policy
Bridion (sugammadex)
Effective June 25, 2021, Bridion (sugammadex) is covered for the following FDA-approved indications:
Bridion is indicated for the reversal of neuromuscular blockade induced by rocuronium bromide and vecuronium bromide in adults and pediatric patients age 2 and older undergoing surgery.
Dosage information:
- Dosing is based on actual body weight.
- Monitor for twitch responses to determine the timing and dose for Bridion administration.
- Administer as a single bolus injection.
For rocuronium and vecuronium:
- 4 mg/kg is recommended if spontaneous recovery of the twitch response has reached one to two post-tetanic counts, or PTC, and there are no twitch responses to train-of-four, or TOF, stimulation.
- 2 mg/kg is recommended if spontaneous recovery has reached the reappearance of the second twitch in response to TOF stimulation.
For rocuronium only:
- 16 mg/kg is recommended if there is a clinical need to reverse neuromuscular blockade soon (approximately 3 minutes) after administration of a single dose of 1.2 mg/kg of rocuronium. Immediate reversal in pediatric patients hasn’t been studied.
Dosage forms and strengths:
- 200 mg/2 mL (100 mg/mL) in a single-dose vial for bolus injection
- 500 mg/5 mL (100 mg/mL) in a single-dose vial for bolus injection
|
C9399
J9999 |
Basic benefit and medical policy
Rylaze asparaginase erwinia chrysanthemi (recombinant)-rywn
Rylaze asparaginase erwinia chrysanthemi (recombinant)-rywn is considered established when criteria are met, effective June 30, 2021.
Indications and usage:
Rylaze is an asparagine-specific enzyme indicated as a component of a multi-agent chemotherapeutic regimen for the treatment of acute lymphoblastic leukemia and lymphoblastic lymphoma in adult and pediatric patients 1 month or older who have developed hypersensitivity to E. coli-derived asparaginase.
Dosage and administration:
When replacing a long-acting asparaginase product, the recommended dosage of Rylaze is 25 mg/m2, administered intramuscularly every 48 hours.
Dosage forms and strengths:
Injection: 10 mg/0.5 mL solution in a single-dose vial
Rylaze asparaginase erwinia chrysanthemi (recombinant)-rywn isn’t a benefit for URMBT. |
H0031, H0032, H2014, H2019, S5108,** S5111,** 0362T, 0373T, 0373T, 97151,** 97152, 97153,*** 97154,** 97155,*** 97156,** 97157,** 97158**
**May be delivered via telemedicine
***May be delivered via telemedicine when the child meets “Guidelines for Autism Interventions” |
Basic benefit and medical policy
Autism spectrum disorder services
The effectiveness of treatment for autism spectrum disorder has been established. It may be a useful therapeutic option when inclusionary and certificate guidelines are met.
Criteria have been updated, effective Nov. 1, 2021.
Inclusions:
- Full diagnostic criteria for autism spectrum disorder, as published in the most recent edition of the American Psychiatric Association’s Diagnostic and Statistical Manual, are met.
- The maladaptive behavior must affect the child’s personal safety or the safety of others within the child’s environment, or must significantly interfere with the child’s ability to function.
- Services in Michigan must be provided or supervised by one of the following:
- A clinician who is a licensed behavior analyst
- A psychiatrist who has the appropriate training
- A licensed psychologist who has the appropriate education, training and experience
- A person who holds a license, certificate or registration that authorizes them to perform services included in applied behavior analysis
- Services outside of Michigan must be provided by a clinician who meets their state requirements to provide ABA therapy.
- There is a treatment plan that:
- Is child centered
- Defines target behaviors
- Records objective measures of baseline levels and progress
- Identifies and documents specific interventions and techniques
- Documents transitional and discharge plans
Exclusions:
- People who don’t meet the diagnostic criteria based on the most recent criteria by the American Psychiatric Association (i.e., most current version of the Diagnostic and Statistical Manual)
- In Michigan, therapy delivered or supervised by clinicians who are not licensed behavior analysts or those who don’t meet state requirements to provide ABA therapy
- Outside of Michigan, therapy delivered or supervised by clinicians who don’t meet their state requirements to provide ABA therapy
- Therapy for people older than age 18
Autism services allowed via telemedicine synchronous care:
- Specific autism services allowed via telemedicine synchronous care are noted in the CPT section.
- Adaptive behavior treatment that is part of a treatment plan (code *97153) is allowed if the child meets appropriateness criteria.
- At a minimum, the child should exhibit basic skills of joint attention, basic discrimination, basic echoic and basic motor imitation. The child should be able to follow common one-step instructions, participate in sessions with limited caregiver assistance and sit independently at a computer or tablet for 8 to 10 minutes. Safety concerns and challenging behaviors must be minimal.
(For the complete guidelines, refer to the Blue Cross’ Provider Alert “Additional autism interventions now payable via telemedicine
on an ongoing basis and restrictions are removed from protocol modification (*97155).” To find the alert, go to bcbsm.com and click on Learn more after What you need to know about the coronavirus, COVID-19. Then click on the Health Care Providers tab. The guidelines are under the Read our Provider Alerts for recent updates section.)
- Autism services provided via telemedicine may not be effective for all children. When services are provided via telemedicine and the child doesn’t show progress, it’s expected that the treatment plan would be modified to face-to-face interactions.
Autism services delivered via telemedicine are synchronous care only; asynchronous care isn’t appropriate for autism services. |
J3490
J3590 |
Basic benefit and medical policy
Aduhelm (aducanumab-avwa)
Aduhelm (aducanumab-avwa) is considered experimental. This policy is effective July 7, 2021. |
J9271 |
Basic benefit and medical policy
Keytruda (pembrolizumab)
Effective June 28, 2021, Keytruda (pembrolizumab) is covered for the following updated FDA-approved indications:
Keytruda (pembrolizumab) is a programmed death receptor-1 (PD-1)-blocking antibody indicated for:
Cutaneous squamous cell carcinoma, or cSCC,
for the treatment of patients with recurrent or metastatic cSCC or locally advanced cSCC that isn’t curable by surgery or radiation.
Dosage and administration:
- cSCC: 200 mg every three weeks or 400 mg every six weeks
|
L5000- L9900,** V2623- V2629, V2630- V2632
**Refer to the appropriate Medicare administrative contractor, or MAC, article for billing guidance |
Basic benefit and medical policy
Prosthetic devices
The safety and effectiveness of prosthetic devices have been established. They may be considered useful therapeutic devices when prescribed by a qualified professional provider to replace absent or nonfunctioning parts of the human body with an artificial substitute, whether surgically implanted or worn as an anatomic supplement. Specific certificate exclusions may apply.
Inclusionary and exclusionary criteria have been updated, effective Nov. 1, 2021.
Inclusions:
Prosthetic appliances include, but are not limited to:
- Internal or surgically implanted permanent prosthesis and external prosthesis to replace all or part of a permanently inoperative or malfunctioning body organ, e.g., artificial joints necessary for joint repair or reconstructive surgery
- Breast forms, internal and external (including a surgical brassiere) for post-mastectomy reconstruction
- Cardiac pacemakers, atomic or electronic
- Intra-ocular lenses as replacement of either surgically removed or congenitally absent crystalline lenses of the eye
- Artificial eyes
- Artificial limbs replacing all or part of absent extremities
- Speech aids
- Urinary collection and retention systems (e.g., Foley catheters, tubing and collection bags) in cases of permanent urinary dysfunction (e.g., incontinence retention)
- Auditory brain stem implants
The prosthetic device must:
- Be prescribed by a qualified health care provider
- Meet the Medicare definition of a prosthetic (i.e., used to replace specific parts of the body or the functions of a permanently inoperative or malfunctioning body part or organ)
Repair or replacement of prosthetic devices may be appropriate when indicated for:
- Repairs and adjustments for preparatory prostheses
- Repairs to make the prosthesis functional
- Repairs or replacement due to a change in the patient’s physiological condition
- Irreparable wear or damage
- Maintenance which may be necessitated by the manufacturer’s recommendations and must be performed by the prosthetist
Exclusions:
Excluded prosthetic devices include, but are not limited to:
- Hearing aids (refer to the Bone-Anchored Hearing Device policy)
- Garter belts
- Dental appliances
- Experimental or research devices
- Appliances used strictly for cosmetic purposes
- Penile prostheses for psychogenic impotence
Note: Check individual contract and certificate language regarding repair, replacement or adjustment of medically appropriate prosthetic devices that is necessitated by wear, damage or medical condition changes.
While traveling: Prosthetic devices are covered when the individual is traveling or staying at another location for a specified period of time. Check individual contract and certificate language, and any specific medical policy related to the item. Repair, replacement or adjustment are also covered as defined by the contract and certificate language and the applicable medical policy. |
None of the information included in this billing chart is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.

Medicare sequestration suspension extended through March 31, 2022
As you may recall, Blue Cross Blue Shield of Michigan and Blue Care Network aligned with the Centers for Medicare & Medicaid Services’ guidance when Congress and the Biden administration suspended the mandatory Medicare 2% sequestration reduction through the end of 2021.
Congress passed legislation on Dec. 9, 2021, that suspends the 2% sequestration reduction through March 31, 2022, and then reduces the sequestration cuts to 1% from April through June 2022. We’ll update you before July 2022 on the status of sequestration after June 30, 2022.
Reminder: The 2% reimbursement adjustment is applied after determining any applicable member deductible, copayment or other required member out-of-pocket costs. The change won’t affect reimbursement to providers who haven’t been affected by sequestration previously, such as providers of durable medical equipment, lab services providers and providers treating patients with end-stage renal disease.
Expansion of existing quantity limit includes new prescriptions for long‑acting opioids and tramadol
Blue Cross Blue Shield of Michigan and Blue Care Network are expanding the existing quantity limits on opioid medications to include long-acting opioids and tramadol. This expansion is in support of the Food and Drug Administration’s efforts to balance the serious risk of opioids with the drugs’ pain management benefits.
Starting Jan. 1, 2022, long-acting opioids and tramadol will have a five-day, first fill limit for commercial members with a new long-acting opioid or tramadol prescription. This change doesn’t apply to members currently taking a long-acting opioid or tramadol, or who are on Medicare.
Improved commercial utilization management medical drug list now available
We’ve published updated documents with utilization management information about drugs covered under the medical benefit for Blue Cross Blue Shield of Michigan and Blue Care Network members. We’ve made changes to the documents, based on feedback from providers and others, to make the information more accessible, clear and streamlined.
The redesigned Blue Cross and BCN utilization management medical drug list:
- Offers a fuller explanation of our medical drug utilization management programs for commercial members
- Indicates more clearly where to submit prior authorization requests — to AIM Specialty Health® or through the NovoLogix® online tool
- Specifies which drugs have prior authorization and site-of-care requirements
- Shows the preferred and nonpreferred products for drugs that we’ve designated as preferred products
- No longer contains medical policy information or documentation requirements information, which makes the list shorter and easier to use
The quantity limits information is in a separate document titled Blue Cross and BCN quantity limits for medical drugs. Here are highlights:
- It provides easier access for providers who need only the quantity limits.
- It indicates whether the quantity limits apply to in-state or out-of-state providers or both.
- The Blue Cross and BCN utilization management medical drug list includes a link to the Blue Cross and BCN quantity limits for medical drugs document in the introductory text and in the table heading on each page.
We make these lists available at ereferrals.bcbsm.com:
You’ll also be able to find these lists behind the provider portal.
We appreciate the feedback we received from the provider community, and welcome additional comments on the new documents. Blue Cross and BCN are committed to providing reliable, up-to-date, easy-to-use resources to help you navigate the medical-benefit drug utilization management programs we have in place.
Changes to prior authorization list for medical benefit drugs for Medicare Advantage members
We’ve added or removed prior authorization requirements for certain drugs for Medicare Plus Blue℠ and BCN Advantage℠ members as follows.
Additional drug requiring prior authorization
For dates of service on or after Dec. 27, 2021, Susvimo™ (ranibizumab injection, for ocular implant), HCPCS code J3590, requires prior authorization through the NovoLogix® online tool.
NovoLogix offers real-time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to NovoLogix. If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
We require prior authorization for this drug when it’s administered by a health care professional in a provider office, at the member’s home, in an off-campus or on-campus outpatient hospital or in an ambulatory surgical center (place of service codes 11, 12, 19, 22 and 24) and billed as follows:
- Electronically through an 837P transaction or on a professional CMS-1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
Drug that no longer requires prior authorization
For dates of service on or after Dec. 1, 2021, Tegsedi® (inotersen), HCPCS code J3490, no longer requires prior authorization.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, please see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
The list was updated to reflect these changes.
Changes coming to medical oncology prior authorization list for URMBT members with Blue Cross non‑Medicare plans
For dates of service on or after Feb. 21, 2022, additional drugs will require prior authorization and some drugs will no longer require prior authorization for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans.
Additional drugs to require prior authorization
For dates of service on or after Feb. 21, 2022, we’re adding prior authorization requirements for the following drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans:
- Khapzory™ (levoleucovorin), HCPCS code J0642
- Neupogen® (filgrastim), HCPCS code J1442
Submit prior authorization requests through AIM Specialty Health® using one of the following methods:
Notes:
- Prior authorization requirements apply when these drugs are administered in an outpatient setting.
- This requirement doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714).
- AIM is an independent company that contracts with Blue Cross Blue Shield of Michigan to provide benefit management services.
Drugs that will no longer require prior authorization
For dates of service on or after Feb. 21, 2022, we’re removing prior authorization requirements for the following drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans:
- Nivestym® (filgrastim-aafi), HCPCS code Q5110
- Udenyca® (pegfilgrastim-cbqv), HCPCS code Q5111
- Ziextenzo® (pegfilgrastim-bmez), HCPCS code Q5120
More about authorization requirements
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, see:
We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date.
Note: Accredo, an independent company that works with the URMBT on specialty pharmacy services, manages prior authorization requests for additional medical benefit drugs.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Radiology procedure code *71271 will require prior authorization for most Blue Cross, BCN members
AIM Specialty Health® currently requires prior authorization for radiology procedure code *71271 for most Blue Cross Blue Shield of Michigan commercial members. Effective with dates of service on or after March 1, 2022, AIM will also require prior authorization for radiology procedure code *71271 for the following health plans:
- Medicare Plus Blue℠
- Blue Care Network commercial
- BCN Advantage℠
Prior authorization will be required to ensure that claims are eligible for reimbursement.
Submitting prior authorization requests
Submit prior authorization requests to AIM. For information on how to submit requests and for other resources, visit these webpages on our ereferrals.bcbsm.com website:
We’ve updated the list of procedures that require prior authorization by AIM to reflect this requirement.
Additional information
As a reminder, AIM manages authorizations for various Blue Cross commercial, Medicare Plus Blue, BCN commercial and BCN Advantage members for these services:
- Select cardiology and radiology services
- Medical oncology and supportive care drugs
- High-tech radiology
- In-lab sleep management
- Radiation oncology
AIM Specialty Health is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services.
Reminder: Our transition to OptumRx
Blue Cross Blue Shield of Michigan and Blue Care Network are transitioning to a new pharmacy benefit manager, moving from Express Scripts, Inc. to OptumRx. This change will take place on Jan. 1, 2022, for commercial individual and group members, and Jan. 1, 2023, for Medicare Advantage individual and group members.
We anticipate the bulk of the transition will be seamless for our members and health care providers. However, members using our current home delivery pharmacy to fill a prescription for a controlled substance — or those with expired prescriptions or prescriptions without refills — should ask their doctor to write a new prescription so it can be filled by OptumRx home delivery pharmacy, starting Jan. 1.
As part of the transition, we’ve mailed about 1.8 million new ID cards to members. Members must show their new cards at the pharmacy starting Jan. 1 to help ensure their prescriptions are covered correctly under their benefits.
We’ve also made some enhancements to our provider-facing tools to assist with prescribing and submitting prior authorizations electronically.
These enhancements will primarily take place behind the scenes and won’t have a major effect on how providers prescribe and submit prior authorizations or check on patients’ benefits.
Continue to use your current electronic medical record system or CoverMyMeds® to submit electronic prior authorizations for Blue Cross and BCN members. Keep in mind that the BIN number changes to 610011, effective Jan. 1, 2022, for all Blue Cross and BCN commercial members.
Need more information?
- For more information on ePA and CoverMyMeds, see our ePA flyer.
- For more information on the transition to OptumRx, see the September issue of The Record and the November-December issue of Hospital and Physician Update.
Here are the 2022 FEP Blue Cross and Blue Shield Service Benefit Plan benefit changes
Blue Cross and Blue Shield Federal Employee Program® Service Benefit Plan 2022 benefit changes will take effect Jan. 1, 2022. Below is an overview of the benefit changes.
Breast pump
- Standard Option, Basic Option and FEP Blue Focus
- The selection of breast pumps is expanding. Each breast pump will come with a selection of milk storage bags.
Dental – accidental injury
- Basic Option
- Coinsurance of 30% for all covered dental codes except for the oral examination to promptly repair injury.
Oral/maxillofacial surgery
- Standard Option and Basic Option
- Prior approval is no longer needed for surgery to correct accidental injuries to jaws, cheeks, lips, tongue, roof and floor of mouth for care provided within 72 hours of the accidental injury.
EKG
- Standard Option, Basic Option and FEP Blue Focus
- EKGs will be covered under the regular medical benefits instead of preventive benefits.
Facility
- Standard Option
- For admission to a participating or non-participating facility due to a medical emergency or accidental injury, copayment is reduced to $350 per admission for unlimited days, then benefits cover 100% of the plan allowance.
Gender reassignment surgery
- Standard Option, Basic Option and FEP Blue Focus
- Reconstruction of the nipple after a mastectomy for female to male gender reassignment surgery is covered.
Group counseling
- Standard Option, Basic Option and FEP Blue Focus
- Group counseling for prevention and reduction of health risks, and group nutritional counseling will be covered under the preventive benefit, with no out-of-pocket cost to members who see a Preferred provider.
Pharmacy
- Standard Option, Basic Option and FEP Blue Focus
- Tubeless insulin pumps covered under the Tier 2 and Tier 3 pharmacy benefits for Standard and Basic Option members. FEP Blue Focus members will pay a Tier 2 copayment.
- Specialty Pharmacy Program will be administered by CVS Caremark®.
- Standard Option only
- Basic Option only
- Copayment changes for members without Medicare Part B
- Tier 4 Preferred specialty drug copayment is now $85 and limited to one purchase of up to a 30-day supply at a Preferred retail pharmacy. All refills must be obtained through the Specialty Pharmacy Program.
- Tier 5 Non-preferred specialty drug copayment is now $110 and limited to one purchase of up to a 30-day supply at a Preferred retail pharmacy. All refills must be obtained through the Specialty Pharmacy Program.
- Copayment changes for members with Medicare Part B primary
- Tier 4 Preferred specialty drug copayment is now $80 and limited to one purchase of up to a 30-day supply at a Preferred retail pharmacy. All refills must be obtained through the Specialty Pharmacy Program.
- Tier 5 Non-preferred specialty drug copayment is now a $100 and limited to one purchase of up to a 30-day supply at a Preferred retail pharmacy. All refills must be obtained through the Specialty Pharmacy Program.
- Updates made to the approved drug lists
- FEP Blue Focus only
- Walgreens® and Duane Reade® pharmacies are no longer in network.
- Members must use in-network pharmacies for their pharmacy benefit.
- Updates made to the approved drug lists
Transplants – Blue Distinction® Centers
- Standard Option and Basic Option
- Kidney transplants now part of the Blue Distinction Centers for Transplant Program and require prior approval.
- Standard Option, Basic Option and FEP Blue Focus
- Pancreas transplants will no longer be part of the Blue Distinction Centers for Transplants Program.
For complete 2022 Blue Cross and Blue Shield Service Benefit Plan benefit information, go to www.fepblue.org/brochure or call Customer Service at 1-800-482-3600.
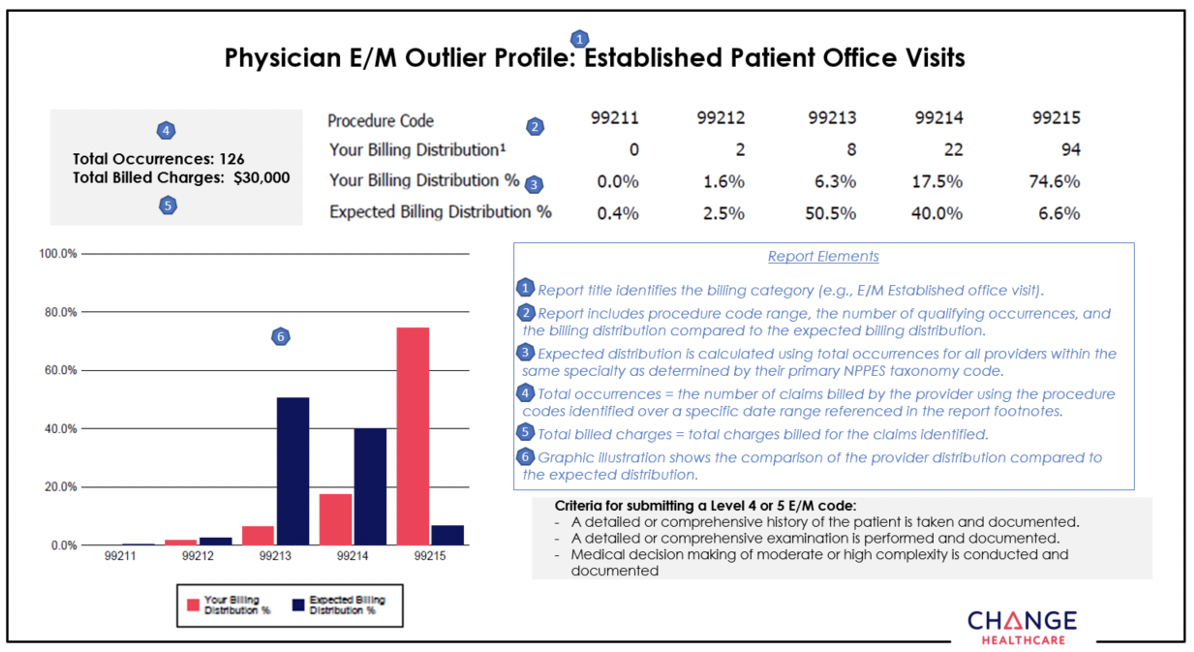
Coding Advisor continuing outreach to educate providers about appropriate use of procedure codes
What you need to know
In January, Change Healthcare will reach out by phone or letter to health care providers who submit claims to Blue Cross and BCN. Coding Advisor will compare the billing of CPT codes to the codes used by a provider’s peers through a physician profile.
It can be challenging for health care providers and their office staff to select the Current Procedural Terminology, or CPT®, code that best reflects the complexity of a patient visit. That’s why Blue Cross Blue Shield of Michigan contracted with Change Healthcare, an independent company, to implement our Coding Advisor program in 2019.
Change Healthcare reviews evaluation and management codes billed and other scenarios — such as modifier 25, observation care and nursing facility care — on claims submitted to Blue Cross. The program provides useful data insights to the provider community and works to maximize coding efficiency and accuracy through up-front education, rather than a traditional postclaim review process.
Effective Jan. 1, 2022, the Coding Advisor program will expand to include Blue Care Network and BCN Advantage℠. While Change Healthcare won’t review E/M services for BCN and BCN Advantage because they use a repricing program that’s already in place, the company will review other modules that include services provided by BCN and BCN Advantage
In January, Change Healthcare will reach out by phone or letter to providers who submit claims to Blue Cross and BCN. Coding Advisor will compare the billing of CPT codes to the codes used by a provider’s peers through a physician profile.
For your reference, we’ve included an example of a physician profile at the end of this article.
Throughout the course of this program, Coding Advisor will continue to monitor billing practices and send updated reports periodically. It may contact your practice to discuss coding variances and to offer one-on-one coding education. You’ll receive all correspondence from Change Healthcare.
If you have any questions, call the Coding Advisor customer support line at
1-844-592-7009 and select option 3.
Example of a physician profile:
New on‑demand training available
Action item
Visit our provider training site to find new resources on topics that are important to your role.
Provider Experience continues to offer training resources for health care providers and staff. Our online, on-demand provider training courses are designed to help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network.
We recently added two new webinars to our list of 2021 lunch and learn webinar recordings:
- Coding Scenarios for Primary Care and Specialty — This webinar shares best practices and a detailed scenario review for common coding errors.
- Evaluation and Management Coding Tips — This webinar focuses on best practices and coding tips for 2021 E&M changes.
We also added an updated online course:
Provider Enrollment and Self-service Tool — This course consists of 16 modules that walk you through the major functions and tasks of this tool.
To request access to our training site, follow these steps:
- Open the registration page.
- Complete the registration. We recommend using the same email you use to communicate with Blue Cross for provider-related needs. This will become your login ID.
- Follow the link to log in.
If you need assistance creating your login ID or navigating the site, contact ProviderTraining@bcbsm.com.
Reminder: Register for Transitions of Care HEDIS measure webinar
Action item
Watch a short video explaining Transitions of Care — and register for a webinar.
As we told you in the December 2021 Record, we’re introducing a new Medicare Star Ratings measure: the HEDIS® measure called Transitions of Care. The measure has four key components. This short video will explain the measure and help you avoid gaps in care.
Register and attend a webinar
Learn more about Transitions of Care, including requirements and coding tips. To register, click on a sign-up link below.
HEDIS® is a registered trademark of the National Committee for Quality Assurance.
Remember to conduct annual wellness visits for your Medicare Advantage patients
Blue Cross Blue Shield of Michigan and Blue Care Network cover annual wellness visits with no out-of-pocket costs for Medicare Plus Blue℠ and BCN Advantage℠ individual and group members. Members are eligible for an annual wellness visit if they’ve had Medicare Part B for longer than 12 months.
These visits involve developing or updating a personalized prevention plan services and conducting a health risk assessment for your patients. Providers can complete the annual wellness visit in the following locations: provider’s office, outpatient hospital or a patient’s home.
Services include:
- An age- and gender-appropriate physical exam (including vital signs and measurements) and a cognitive assessment
- Guidance, counseling and risk-factor reduction interventions
- Administration or ordering of immunizations, lab tests or diagnostic procedures
Blue Cross recognizes the importance of preventive care to our members' health and encourages providers to discuss with their patients the risk factors identified in the assessments.
For additional information, including how to bill the initial and subsequent annual wellness visits, refer to Medicare Wellness Visits,** on the Centers for Medicare & Medicaid Services’ Medicare Learning Network webpage.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Reminder: Optum provides claim editing for professional and facility claims
In November 2021, Optum began editing commercial claims for Blue Cross Blue Shield of Michigan professional and facility providers. We initially informed you of our partnership with Optum for enhanced claim editing in the August edition of The Record.
Health care providers will see these new edits as we continue our ongoing effort to promote correct coding and enhance our claims payment systems. You'll be able to recognize the edits by the unique provider message codes K500 through K544.
The Optum medical records request component of this initiative is expected to begin during the first quarter of 2022.
If you receive one of these edits, submit a corrected claim when appropriate. Providers should continue to follow the current clinical editing appeals process if they believe the services rendered warrant an exception.
Reminder: Update Provider Authorization form when changes occur
Blue Cross Blue Shield of Michigan is dedicated to safeguarding the protected health information of its members. These safeguards include completion of a Trading Partner Agreement and Provider Authorization form as part of the electronic data interchange setup process. All EDI trading partners must complete a TPA and Provider Authorization form before they can exchange PHI with Blue Cross.
The terms of the TPA require you to notify Blue Cross of any changes in your trading partner information, so it’s important to keep your Provider Authorization form updated. Updating the form also ensures information is routed to the proper destination.
Update the form when you decide you no longer want to receive 835 remittance files or when you switch:
- Service bureaus or clearinghouses
- Software vendors
- Billing services
- Submitter IDs
- 835 file recipients
- Unique 835 receivers or trading partner IDs
Review the form when you:
- Join a new group practice.
- Leave a group practice and start billing using your own NPI.
- Hire a new billing service.
- Start submitting claims through a clearinghouse or when you’ve changed clearinghouses.
- Select a new destination for your 835.
You don’t need to update the Provider Authorization form if your submitter and trading partner IDs don’t change.
How to change your EDI setup
To make changes to your EDI setup log in to the Trading Partner Agreement webpage.
Or follow the complete navigation steps to arrive at the TPA login page:
- Visit bcbsm.com/providers.
- Scroll down to the Provider resources section and click on Quick Links.
- Click on Electronic Connectivity EDI.
- Click on How to use EDI to exchange information with us electronically.
- Scroll down to the EDI agreements section and click on Update your Provider Authorization Form.
If you have any questions about EDI enrollment, contact the EDI Help Desk at EDISupport@bcbsm.com. Include your billing NPI and submitter ID with all correspondence.

Improved commercial utilization management medical drug list now available
We’ve published updated documents with utilization management information about drugs covered under the medical benefit for Blue Cross Blue Shield of Michigan and Blue Care Network members. We’ve made changes to the documents, based on feedback from providers and others, to make the information more accessible, clear and streamlined.
The redesigned Blue Cross and BCN utilization management medical drug list:
- Offers a fuller explanation of our medical drug utilization management programs for commercial members
- Indicates more clearly where to submit prior authorization requests — to AIM Specialty Health® or through the NovoLogix® online tool
- Specifies which drugs have prior authorization and site-of-care requirements
- Shows the preferred and nonpreferred products for drugs that we’ve designated as preferred products
- No longer contains medical policy information or documentation requirements information, which makes the list shorter and easier to use
The quantity limits information is in a separate document titled Blue Cross and BCN quantity limits for medical drugs. Here are highlights:
- It provides easier access for providers who need only the quantity limits.
- It indicates whether the quantity limits apply to in-state or out-of-state providers or both.
- The Blue Cross and BCN utilization management medical drug list includes a link to the Blue Cross and BCN quantity limits for medical drugs document in the introductory text and in the table heading on each page.
We make these lists available at ereferrals.bcbsm.com:
You’ll also be able to find these lists behind the provider portal.
We appreciate the feedback we received from the provider community, and welcome additional comments on the new documents. Blue Cross and BCN are committed to providing reliable, up-to-date, easy-to-use resources to help you navigate the medical-benefit drug utilization management programs we have in place.
Changes to prior authorization list for medical benefit drugs for Medicare Advantage members
We’ve added or removed prior authorization requirements for certain drugs for Medicare Plus Blue℠ and BCN Advantage℠ members as follows.
Additional drug requiring prior authorization
For dates of service on or after Dec. 27, 2021, Susvimo™ (ranibizumab injection, for ocular implant), HCPCS code J3590, requires prior authorization through the NovoLogix® online tool.
NovoLogix offers real-time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to NovoLogix. If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
We require prior authorization for this drug when it’s administered by a health care professional in a provider office, at the member’s home, in an off-campus or on-campus outpatient hospital or in an ambulatory surgical center (place of service codes 11, 12, 19, 22 and 24) and billed as follows:
- Electronically through an 837P transaction or on a professional CMS-1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
Drug that no longer requires prior authorization
For dates of service on or after Dec. 1, 2021, Tegsedi® (inotersen), HCPCS code J3490, no longer requires prior authorization.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, please see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
The list was updated to reflect these changes.
Changes coming to medical oncology prior authorization list for URMBT members with Blue Cross non‑Medicare plans
For dates of service on or after Feb. 21, 2022, additional drugs will require prior authorization and some drugs will no longer require prior authorization for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans.
Additional drugs to require prior authorization
For dates of service on or after Feb. 21, 2022, we’re adding prior authorization requirements for the following drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans:
- Khapzory™ (levoleucovorin), HCPCS code J0642
- Neupogen® (filgrastim), HCPCS code J1442
Submit prior authorization requests through AIM Specialty Health® using one of the following methods:
Notes:
- Prior authorization requirements apply when these drugs are administered in an outpatient setting.
- This requirement doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714).
- AIM is an independent company that contracts with Blue Cross Blue Shield of Michigan to provide benefit management services.
Drugs that will no longer require prior authorization
For dates of service on or after Feb. 21, 2022, we’re removing prior authorization requirements for the following drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans:
- Nivestym® (filgrastim-aafi), HCPCS code Q5110
- Udenyca® (pegfilgrastim-cbqv), HCPCS code Q5111
- Ziextenzo® (pegfilgrastim-bmez), HCPCS code Q5120
More about authorization requirements
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, see:
We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date.
Note: Accredo, an independent company that works with the URMBT on specialty pharmacy services, manages prior authorization requests for additional medical benefit drugs.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Radiology procedure code *71271 will require prior authorization for most Blue Cross, BCN members
AIM Specialty Health® currently requires prior authorization for radiology procedure code *71271 for most Blue Cross Blue Shield of Michigan commercial members. Effective with dates of service on or after March 1, 2022, AIM will also require prior authorization for radiology procedure code *71271 for the following health plans:
- Medicare Plus Blue℠
- Blue Care Network commercial
- BCN Advantage℠
Prior authorization will be required to ensure that claims are eligible for reimbursement.
Submitting prior authorization requests
Submit prior authorization requests to AIM. For information on how to submit requests and for other resources, visit these webpages on our ereferrals.bcbsm.com website:
We’ve updated the list of procedures that require prior authorization by AIM to reflect this requirement.
Additional information
As a reminder, AIM manages authorizations for various Blue Cross commercial, Medicare Plus Blue, BCN commercial and BCN Advantage members for these services:
- Select cardiology and radiology services
- Medical oncology and supportive care drugs
- High-tech radiology
- In-lab sleep management
- Radiation oncology
AIM Specialty Health is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services.
Here are the 2022 FEP Blue Cross and Blue Shield Service Benefit Plan benefit changes
Blue Cross and Blue Shield Federal Employee Program® Service Benefit Plan 2022 benefit changes will take effect Jan. 1, 2022. Below is an overview of the benefit changes.
Breast pump
- Standard Option, Basic Option and FEP Blue Focus
- The selection of breast pumps is expanding. Each breast pump will come with a selection of milk storage bags.
Dental – accidental injury
- Basic Option
- Coinsurance of 30% for all covered dental codes except for the oral examination to promptly repair injury.
Oral/maxillofacial surgery
- Standard Option and Basic Option
- Prior approval is no longer needed for surgery to correct accidental injuries to jaws, cheeks, lips, tongue, roof and floor of mouth for care provided within 72 hours of the accidental injury.
EKG
- Standard Option, Basic Option and FEP Blue Focus
- EKGs will be covered under the regular medical benefits instead of preventive benefits.
Facility
- Standard Option
- For admission to a participating or non-participating facility due to a medical emergency or accidental injury, copayment is reduced to $350 per admission for unlimited days, then benefits cover 100% of the plan allowance.
Gender reassignment surgery
- Standard Option, Basic Option and FEP Blue Focus
- Reconstruction of the nipple after a mastectomy for female to male gender reassignment surgery is covered.
Group counseling
- Standard Option, Basic Option and FEP Blue Focus
- Group counseling for prevention and reduction of health risks, and group nutritional counseling will be covered under the preventive benefit, with no out-of-pocket cost to members who see a Preferred provider.
Pharmacy
- Standard Option, Basic Option and FEP Blue Focus
- Tubeless insulin pumps covered under the Tier 2 and Tier 3 pharmacy benefits for Standard and Basic Option members. FEP Blue Focus members will pay a Tier 2 copayment.
- Specialty Pharmacy Program will be administered by CVS Caremark®.
- Standard Option only
- Basic Option only
- Copayment changes for members without Medicare Part B
- Tier 4 Preferred specialty drug copayment is now $85 and limited to one purchase of up to a 30-day supply at a Preferred retail pharmacy. All refills must be obtained through the Specialty Pharmacy Program.
- Tier 5 Non-preferred specialty drug copayment is now $110 and limited to one purchase of up to a 30-day supply at a Preferred retail pharmacy. All refills must be obtained through the Specialty Pharmacy Program.
- Copayment changes for members with Medicare Part B primary
- Tier 4 Preferred specialty drug copayment is now $80 and limited to one purchase of up to a 30-day supply at a Preferred retail pharmacy. All refills must be obtained through the Specialty Pharmacy Program.
- Tier 5 Non-preferred specialty drug copayment is now a $100 and limited to one purchase of up to a 30-day supply at a Preferred retail pharmacy. All refills must be obtained through the Specialty Pharmacy Program.
- Updates made to the approved drug lists
- FEP Blue Focus only
- Walgreens® and Duane Reade® pharmacies are no longer in network.
- Members must use in-network pharmacies for their pharmacy benefit.
- Updates made to the approved drug lists
Transplants – Blue Distinction® Centers
- Standard Option and Basic Option
- Kidney transplants now part of the Blue Distinction Centers for Transplant Program and require prior approval.
- Standard Option, Basic Option and FEP Blue Focus
- Pancreas transplants will no longer be part of the Blue Distinction Centers for Transplants Program.
For complete 2022 Blue Cross and Blue Shield Service Benefit Plan benefit information, go to www.fepblue.org/brochure or call Customer Service at 1-800-482-3600.
Coming later in 2022: Coding update for Medicare Plus Blue outpatient emergency department claims
Blue Cross Blue Shield of Michigan will enhance its claim editing process later this year for evaluation and management services on outpatient facility emergency department claims for Medicare Plus Blue℠ members. We’re making this update to promote correct coding and assist with payment accuracy.
This coding update focuses on outpatient facility emergency department claims that are submitted with Level 4 (*99284, G0383) or Level 5 (*99285, G0384) E/M codes. The process was developed to address inconsistencies in coding accuracy nationwide.
It’s based on the E/M coding principles developed by the Centers for Medicare & Medicaid Services. These principles help ensure that hospital emergency department E/M coding guidelines follow the intent of Current Procedural Terminology, or CPT®, code descriptions and relate to hospital resource use.
CPT code levels:
- *99281 — ER visit for the evaluation and management of a patient (Level 1)
- *99282 — ER visit for the evaluation and management of a patient (Level 2)
- *99283 — ER visit for the evaluation and management of a patient (Level 3)
- *99284 — ER visit for the evaluation and management of a patient (Level 4)
- *99285 — ER visit for the evaluation and management of a patient (Level 5)
This coding update will apply to all facilities, including freestanding facilities, that submit outpatient emergency department claims with Level 4 or Level 5 E/M codes. The facilities may experience adjustments to the submitted code to reflect an appropriate-level E/M code.
Optum EDC Analyzer™ tool
As part of the implementation of this process, we’ll begin using the Optum EDC Analyzer tool. The tool determines appropriate E/M coding levels based on data from a patient’s claim, and includes the following:
- Patient’s presenting problem
- Diagnostic services performed during the visit
- Any of the patient’s complicating conditions
To learn more about the EDC Analyzer tool — and even try running a claim through the tool — visit EDCAnalyzer.com.**
Exclusions
Criteria that may exclude outpatient facility claims from these policies include, but are not limited to:
- Claims for patients who were admitted from the emergency department or transferred to another health care setting (for example: a skilled nursing facility or long-term care hospital)
- Claims for patients who received critical care services (*99291, *99292)
- Claims for patients who are younger than 2 years old
- Claims with certain diagnosis codes that when treated in the emergency department most often necessitate greater-than-average resource usage, such as significant nursing time
- Claims for patients who died in the emergency department
Ultimately, the goal of facility coding is to accurately capture emergency department resource utilization and align that with the E/M CPT® code description for a patient visit per CMS guidance.
Note: The appeal process won’t change. Submitters who believe a higher-level E/M code is justified for the outpatient emergency department visit should send an appeal on the Clinical Editing Appeal Form with the necessary documentation. Remember to continue to fax one appeal at a time to avoid processing delays.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
New billing guidelines for cystourethroscopy procedures
Blue Cross Blue Shield of Michigan has discontinued using cystourethroscopy procedure codes *52441 and *52442. These codes are being turned off and replaced with Level II HCPCS procedure codes C9739 and C9740 to accommodate the appropriate reimbursement for multiple quantities.
Facilities should report C9739 when performing a single implant and C9740 for each additional permanent adjustable implant, along with the applicable quantity.
The effective date of this change is retroactive to Jan. 1, 2020, to accommodate the resubmission of claims where the appropriate reimbursement was affected by multiple quantities. Facilities may resubmit claims processed for dates of service Jan. 1, 2020, through the present.
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
New on‑demand training available
Action item
Visit our provider training site to find new resources on topics that are important to your role.
Provider Experience continues to offer training resources for health care providers and staff. Our online, on-demand provider training courses are designed to help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network.
We recently added two new webinars to our list of 2021 lunch and learn webinar recordings:
- Coding Scenarios for Primary Care and Specialty — This webinar shares best practices and a detailed scenario review for common coding errors.
- Evaluation and Management Coding Tips — This webinar focuses on best practices and coding tips for 2021 E&M changes.
We also added an updated online course:
Provider Enrollment and Self-service Tool — This course consists of 16 modules that walk you through the major functions and tasks of this tool.
To request access to our training site, follow these steps:
- Open the registration page.
- Complete the registration. We recommend using the same email you use to communicate with Blue Cross for provider-related needs. This will become your login ID.
- Follow the link to log in.
If you need assistance creating your login ID or navigating the site, contact ProviderTraining@bcbsm.com.
Reminder: Register for Transitions of Care HEDIS measure webinar
Action item
Watch a short video explaining Transitions of Care — and register for a webinar.
As we told you in the December 2021 Record, we’re introducing a new Medicare Star Ratings measure: the HEDIS® measure called Transitions of Care. The measure has four key components. This short video will explain the measure and help you avoid gaps in care.
Register and attend a webinar
Learn more about Transitions of Care, including requirements and coding tips. To register, click on a sign-up link below.
HEDIS® is a registered trademark of the National Committee for Quality Assurance.
Additional update: We’re aligning local rules for acute inpatient medical admissions
What you need to know
We’ve updated the article on this topic that ran in the December 2021 issue of The Record.
Key changes: We’ve clarified that authorization requests for inpatient admissions for certain acute medical conditions should be submitted only after the member has spent two days in the hospital. (We previously indicated that the member had to be in the hospital for 48 hours.) Also, the update to local rules is now effective for all members admitted on or after March 1, 2022. Use the following updated article as your reference.
For certain conditions, authorization requests for acute medical admissions should be submitted only after the member has spent two days in the hospital. Once two days has elapsed, the facility can submit the request to authorize an inpatient admission on the third day. You must provide clinical documentation that demonstrates that the InterQual® criteria have been met at the time you submit the request.
Exception: When a member is receiving intensive care services that require a critical care setting, you can submit the request prior to completion of the two-day period, along with all clinical documentation supporting the critical level of care.
We’re aligning our local rules for all lines of business to reflect this change.
Effective date for this change
This update to local rules will go into for all members on or after March 1, 2022.
This applies to members with the following conditions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- Skin and soft tissue infection
|
|
|
|
|
- Intractable low back pain
|
- Transient ischemic attack
|
|
How determinations will be made
Blue Cross and BCN will conduct a medical necessity review based on the clinical documentation you submitted. InterQual criteria will be applied based on the member’s condition at the time the clinical documentation is received:
- If InterQual criteria are met, the authorization request will be approved.
- If InterQual criteria aren’t met, the authorization request will be sent to the plan medical director for review.
- If the member hasn’t been in the hospital for 48 hours and isn’t in the ICU, Blue Cross and BCN will request that the facility wait until the member has been in the hospital for 48 hours to send additional information about the member’s condition. We’ll make the request through the Case Communication field in the e-referral system or by calling the facility, or both.
After receiving the request from the hospital on the third day, Blue Cross and BCN will do the following:
- If the facility sent additional clinical information and it meets criteria, we’ll approve the request.
- If the facility hasn’t sent additional clinical information or has sent additional clinical information but it doesn’t meet criteria, we’ll refer the request to the medical director for review.
For requests that are nonapproved, Blue Cross and BCN will reimburse as observation. The hospital will need to submit a claim for observation reimbursement.
Reason for change
We expect that this change will:
- Reduce the number of communications that typically accompany these types of authorization requests.
- Decrease denials for lack of clinical information because all clinical documentation in support of the admission would be received after two days of hospital care.
- Ensure appropriate reimbursement (inpatient versus observation level of care).
Additional information
For most members, facilities can request peer-to-peer reviews, if desired. Refer to the document How to request a peer-to-peer review with a Blue Cross or BCN medical director.
In addition, facilities can appeal denial decisions as usual. Refer to the provider manual for information on how to submit an appeal.
Reminder: Optum provides claim editing for professional and facility claims
In November 2021, Optum began editing commercial claims for Blue Cross Blue Shield of Michigan professional and facility providers. We initially informed you of our partnership with Optum for enhanced claim editing in the August edition of The Record.
Health care providers will see these new edits as we continue our ongoing effort to promote correct coding and enhance our claims payment systems. You'll be able to recognize the edits by the unique provider message codes K500 through K544.
The Optum medical records request component of this initiative is expected to begin during the first quarter of 2022.
If you receive one of these edits, submit a corrected claim when appropriate. Providers should continue to follow the current clinical editing appeals process if they believe the services rendered warrant an exception.
Reminder: Update Provider Authorization form when changes occur
Blue Cross Blue Shield of Michigan is dedicated to safeguarding the protected health information of its members. These safeguards include completion of a Trading Partner Agreement and Provider Authorization form as part of the electronic data interchange setup process. All EDI trading partners must complete a TPA and Provider Authorization form before they can exchange PHI with Blue Cross.
The terms of the TPA require you to notify Blue Cross of any changes in your trading partner information, so it’s important to keep your Provider Authorization form updated. Updating the form also ensures information is routed to the proper destination.
Update the form when you decide you no longer want to receive 835 remittance files or when you switch:
- Service bureaus or clearinghouses
- Software vendors
- Billing services
- Submitter IDs
- 835 file recipients
- Unique 835 receivers or trading partner IDs
Review the form when you:
- Join a new group practice.
- Leave a group practice and start billing using your own NPI.
- Hire a new billing service.
- Start submitting claims through a clearinghouse or when you’ve changed clearinghouses.
- Select a new destination for your 835.
You don’t need to update the Provider Authorization form if your submitter and trading partner IDs don’t change.
How to change your EDI setup
To make changes to your EDI setup log in to the Trading Partner Agreement webpage.
Or follow the complete navigation steps to arrive at the TPA login page:
- Visit bcbsm.com/providers.
- Scroll down to the Provider resources section and click on Quick Links.
- Click on Electronic Connectivity EDI.
- Click on How to use EDI to exchange information with us electronically.
- Scroll down to the EDI agreements section and click on Update your Provider Authorization Form.
If you have any questions about EDI enrollment, contact the EDI Help Desk at EDISupport@bcbsm.com. Include your billing NPI and submitter ID with all correspondence.

Expansion of existing quantity limit includes new prescriptions for long‑acting opioids and tramadol
Blue Cross Blue Shield of Michigan and Blue Care Network are expanding the existing quantity limits on opioid medications to include long-acting opioids and tramadol. This expansion is in support of the Food and Drug Administration’s efforts to balance the serious risk of opioids with the drugs’ pain management benefits.
Starting Jan. 1, 2022, long-acting opioids and tramadol will have a five-day, first fill limit for commercial members with a new long-acting opioid or tramadol prescription. This change doesn’t apply to members currently taking a long-acting opioid or tramadol, or who are on Medicare.
Improved commercial utilization management medical drug list now available
We’ve published updated documents with utilization management information about drugs covered under the medical benefit for Blue Cross Blue Shield of Michigan and Blue Care Network members. We’ve made changes to the documents, based on feedback from providers and others, to make the information more accessible, clear and streamlined.
The redesigned Blue Cross and BCN utilization management medical drug list:
- Offers a fuller explanation of our medical drug utilization management programs for commercial members
- Indicates more clearly where to submit prior authorization requests — to AIM Specialty Health® or through the NovoLogix® online tool
- Specifies which drugs have prior authorization and site-of-care requirements
- Shows the preferred and nonpreferred products for drugs that we’ve designated as preferred products
- No longer contains medical policy information or documentation requirements information, which makes the list shorter and easier to use
The quantity limits information is in a separate document titled Blue Cross and BCN quantity limits for medical drugs. Here are highlights:
- It provides easier access for providers who need only the quantity limits.
- It indicates whether the quantity limits apply to in-state or out-of-state providers or both.
- The Blue Cross and BCN utilization management medical drug list includes a link to the Blue Cross and BCN quantity limits for medical drugs document in the introductory text and in the table heading on each page.
We make these lists available at ereferrals.bcbsm.com:
You’ll also be able to find these lists behind the provider portal.
We appreciate the feedback we received from the provider community, and welcome additional comments on the new documents. Blue Cross and BCN are committed to providing reliable, up-to-date, easy-to-use resources to help you navigate the medical-benefit drug utilization management programs we have in place.
Changes to prior authorization list for medical benefit drugs for Medicare Advantage members
We’ve added or removed prior authorization requirements for certain drugs for Medicare Plus Blue℠ and BCN Advantage℠ members as follows.
Additional drug requiring prior authorization
For dates of service on or after Dec. 27, 2021, Susvimo™ (ranibizumab injection, for ocular implant), HCPCS code J3590, requires prior authorization through the NovoLogix® online tool.
NovoLogix offers real-time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to NovoLogix. If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
We require prior authorization for this drug when it’s administered by a health care professional in a provider office, at the member’s home, in an off-campus or on-campus outpatient hospital or in an ambulatory surgical center (place of service codes 11, 12, 19, 22 and 24) and billed as follows:
- Electronically through an 837P transaction or on a professional CMS-1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
Drug that no longer requires prior authorization
For dates of service on or after Dec. 1, 2021, Tegsedi® (inotersen), HCPCS code J3490, no longer requires prior authorization.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, please see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
The list was updated to reflect these changes.
Changes coming to medical oncology prior authorization list for URMBT members with Blue Cross non‑Medicare plans
For dates of service on or after Feb. 21, 2022, additional drugs will require prior authorization and some drugs will no longer require prior authorization for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans.
Additional drugs to require prior authorization
For dates of service on or after Feb. 21, 2022, we’re adding prior authorization requirements for the following drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans:
- Khapzory™ (levoleucovorin), HCPCS code J0642
- Neupogen® (filgrastim), HCPCS code J1442
Submit prior authorization requests through AIM Specialty Health® using one of the following methods:
Notes:
- Prior authorization requirements apply when these drugs are administered in an outpatient setting.
- This requirement doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714).
- AIM is an independent company that contracts with Blue Cross Blue Shield of Michigan to provide benefit management services.
Drugs that will no longer require prior authorization
For dates of service on or after Feb. 21, 2022, we’re removing prior authorization requirements for the following drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans:
- Nivestym® (filgrastim-aafi), HCPCS code Q5110
- Udenyca® (pegfilgrastim-cbqv), HCPCS code Q5111
- Ziextenzo® (pegfilgrastim-bmez), HCPCS code Q5120
More about authorization requirements
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, see:
We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date.
Note: Accredo, an independent company that works with the URMBT on specialty pharmacy services, manages prior authorization requests for additional medical benefit drugs.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Reminder: Our transition to OptumRx
Blue Cross Blue Shield of Michigan and Blue Care Network are transitioning to a new pharmacy benefit manager, moving from Express Scripts, Inc. to OptumRx. This change will take place on Jan. 1, 2022, for commercial individual and group members, and Jan. 1, 2023, for Medicare Advantage individual and group members.
We anticipate the bulk of the transition will be seamless for our members and health care providers. However, members using our current home delivery pharmacy to fill a prescription for a controlled substance — or those with expired prescriptions or prescriptions without refills — should ask their doctor to write a new prescription so it can be filled by OptumRx home delivery pharmacy, starting Jan. 1.
As part of the transition, we’ve mailed about 1.8 million new ID cards to members. Members must show their new cards at the pharmacy starting Jan. 1 to help ensure their prescriptions are covered correctly under their benefits.
We’ve also made some enhancements to our provider-facing tools to assist with prescribing and submitting prior authorizations electronically.
These enhancements will primarily take place behind the scenes and won’t have a major effect on how providers prescribe and submit prior authorizations or check on patients’ benefits.
Continue to use your current electronic medical record system or CoverMyMeds® to submit electronic prior authorizations for Blue Cross and BCN members. Keep in mind that the BIN number changes to 610011, effective Jan. 1, 2022, for all Blue Cross and BCN commercial members.
Need more information?
- For more information on ePA and CoverMyMeds, see our ePA flyer.
- For more information on the transition to OptumRx, see the September issue of The Record and the November-December issue of Hospital and Physician Update.
|

 This is part of a series of articles on our move to Availity® for our provider portal.
This is part of a series of articles on our move to Availity® for our provider portal.