Subscribe | The Record Archive | Contacts | bcbsm.com
|
November 2021
Blue Cross contributes $5 million to MHA Keystone Center to support research and innovationsBlue Cross Blue Shield of Michigan recently announced that it’s expanding its longstanding relationship with the Michigan Health & Hospital Association Keystone Center with a $5 million contribution. Since the launch of the MHA Keystone Center in 2003, participating hospitals have made significant strides in increasing safety and quality and have been recognized nationally for their work to improve care statewide. This newest investment from Blue Cross, which will be paid in installments through 2024, adds to the $16 million Blue Cross has provided to the MHA Keystone Center since 2009. Funding will directly support new programs and hospital-led innovations related to women and children’s health, maternal care and the safety of both patients and health care workers. The funding will also support Blue Cross and the MHA Keystone Center’s work encouraging Michigan hospitals to offer medication assisted treatment for substance use disorders to help combat the opioid epidemic — an ongoing focus of Blue Cross and the MHA Keystone Center. “Since its creation 18 years ago, the MHA Keystone Center has performed critical work that has positioned Michigan as one of the leading places to receive hospital-based care,” said Blue Cross Blue Shield of Michigan President and CEO Daniel J. Loepp. “Blue Cross and MHA have mutual interest in promoting hospital-based care that is not only safe, but that delivers the positive outcomes patients count on when they first arrive at their community’s hospital.” MHA CEO Brian Peters added: “Delivering safe, high-quality care to every patient every time is at the core of every Michigan hospital’s mission. The investments from BCBSM for the ongoing quality and safety work of the MHA Keystone Center has allowed hospitals across the state to collaborate on issues that directly impact our patients and employees. Together, this work has led to lives saved and health care errors and costs prevented.” Some recent successes
Promoting a safer environment James Grant, Blue Cross senior vice president and chief medical officer, who practiced as an anesthesiologist for more than 30 years, saw the positive effects firsthand. “We do things differently and in a safer manner today because of this work,” he said. “Patient checklists exist today that didn’t exist before the MHA Keystone Center. “Staff take timeouts to assure protocols have been followed. Precautions have increased to promote safety and ensure sterile techniques for various procedures to avoid infections. The MHA Keystone Center has been instrumental in leading to a safer care environment for each patient we serve.” For more information
We’re moving to Availity in 2022We’re excited to share the timeline for our transition to the Availity® provider portal. Here are the main dates:
Don’t worry. We’ll share more information with you along the way, and there will be plenty of opportunities for training when it’s your turn to make the move to Availity. In the meantime, we’re working to ensure that the move to Availity provides you with the features you want and the accuracy and dependability you’re used to. Questions? If you have questions about the move to Availity, check our Frequently Asked Questions document first. If your question isn’t already answered there, submit your question to ProviderPortalQuestions@bcbsm.com so we can consider adding it to the FAQ document.
Previous articles about Availity We’re providing a series of articles focusing on our move to Availity for our provider portal. Here are the articles we’ve already published in case you missed them:
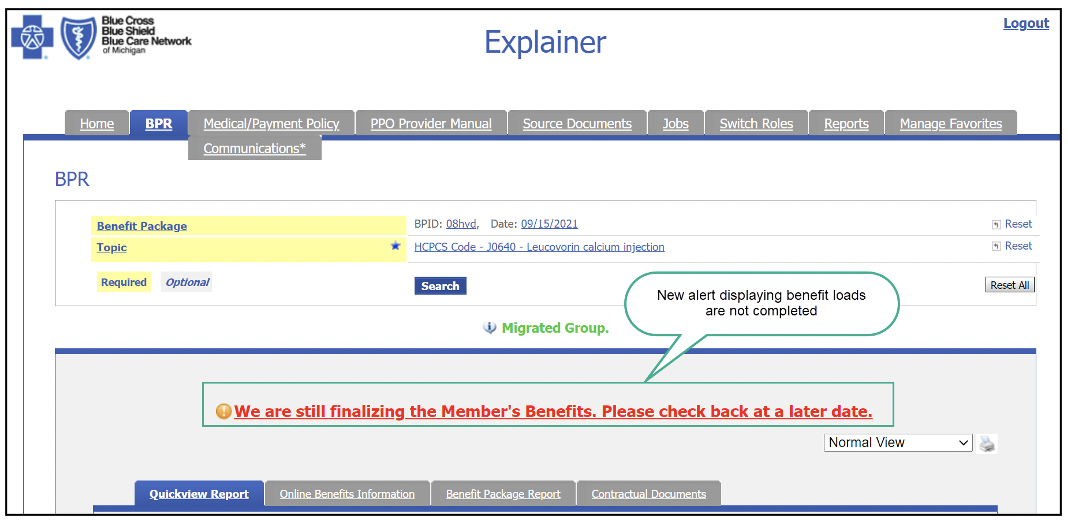
We’ve enhanced Benefit Explainer in several important waysWhat you need to know On Nov. 1, 2021, Blue Cross Blue Shield of Michigan will launch some new features on Benefit Explainer. If a benefit package ID or contract number doesn’t have complete benefits loaded, users will now see a message under the Benefit Package Report tab. The message informs users that there are pending benefit loads and they should check back later. It will appear in red font in the middle of the report screen. Once benefit loads are completed, the message will be removed and no longer will be displayed. The message looks like this:
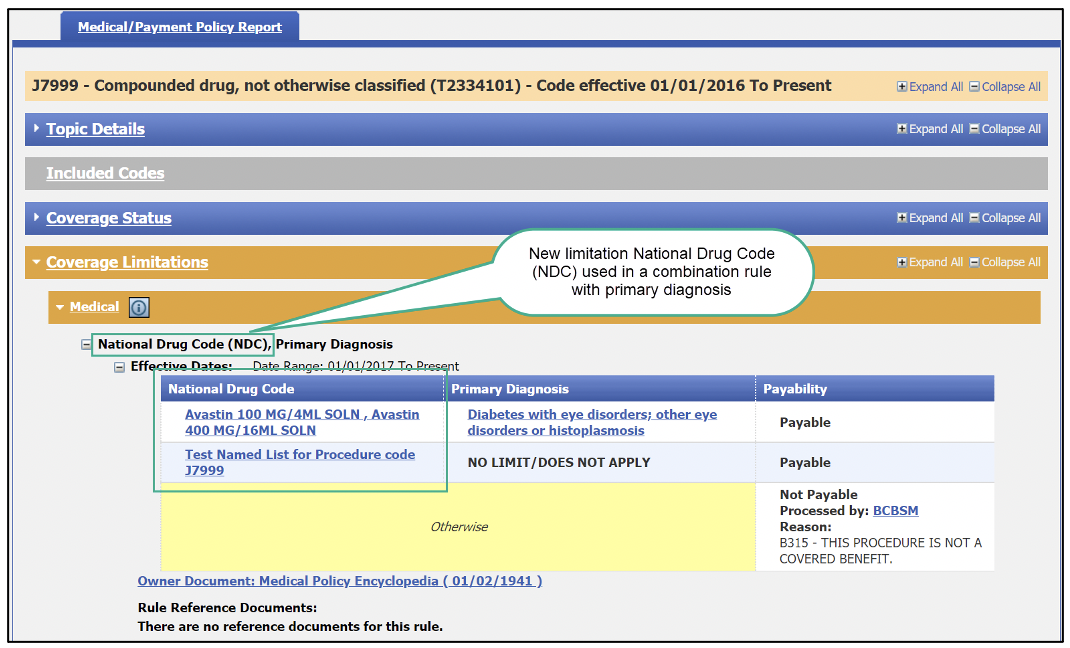
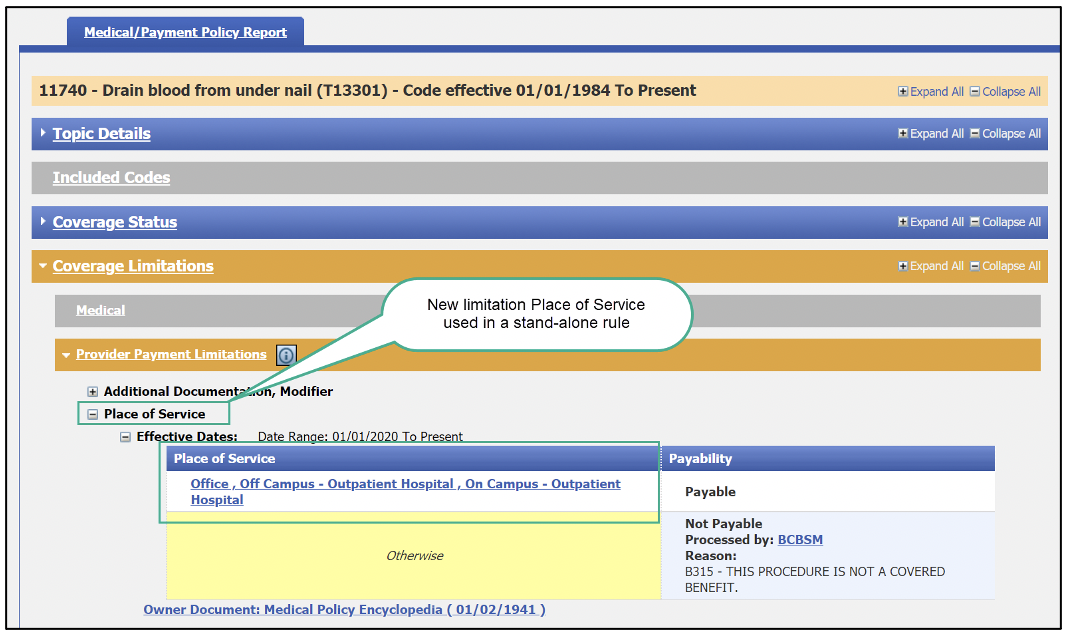
Users will also now see two new “payability limitations” in the Medical/Payment Policy and Benefit Package Report tabs. The limitations are:
The new limitations can appear as a stand-alone rule or as a combination rule with another limitation. They can appear in any of the following fields:
Here are a few screenshots of how this will look:
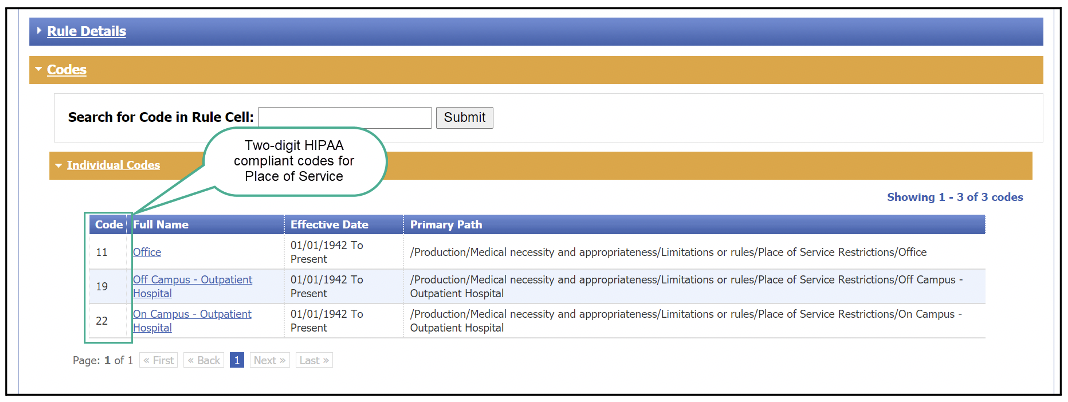
When a user clicks on the link in the Place of Service column, Benefit Explainer will open a new tab. In the new tab, the user can view the two-digit, HIPAA-compliant codes.
Note: Screenshots are for illustrative purposes only and may not represent actual data.
Share your opinions with Blue Cross and BCNBlue Cross Blue Shield of Michigan and Blue Care Network would like to hear from health care providers about their experiences and interactions with us. We strive to make doing business with us as easy as possible, and we ask providers for feedback on our efforts periodically. If you receive an invitation to complete a survey or participate in other types of research, we urge you to give us your opinions. Results are used to identify areas for improvement. You can stay up to date on all opportunities to give feedback at the new Share your opinion with Blue Cross and BCN webpage. On that page, you’ll find current opportunities to share your opinions with us on various topics. To find the webpage, follow these steps:
Clarification: Contacting Provider InquiryIn the October Record, we ran an article stating that if you’re experiencing claims issues, you should contact Provider Inquiry before calling your provider consultant. This information is correct. However, we’ve made a slight adjustment to the first section of the article and are reprinting the changed area below (new information bolded in the excerpt below): To speak with a Provider Inquiry representative, call one of the following phone numbers from 8:30 a.m. to noon and 1 to 5 p.m. Monday through Friday:
If you don’t think your issue has been satisfactorily resolved, ask the representative to escalate your inquiry to our help desk in Provider Inquiry. The October Record article has been updated to reflect this change.
Third‑quarter 2021 CPT code updatePathology and laboratory
Pathology and laboratory
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations. Billing chart: Blue Cross highlights medical, benefit policy changesYou’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart. This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures. You will also see that descriptions for the codes are no longer included. This is a result of recent negotiations with the AMA on use of the codes. We will publish information about new BCBS groups or changes to group benefits under the Group Benefit Changes heading. For more detailed descriptions of the BCBSM policies for these procedures, please check under the Medical/Payment Policy tab in Explainer on web-DENIS. To access this online information:
CareCentrix home health care program: Updated training resources, new and updated documents availableCareCentrix® manages prior authorizations for home health care services for Medicare Plus Blue℠ and BCN Advantage℠ members as follows:
Where to find CareCentrix home health care resources You can find the training resources and links to the documents related to this program on the following pages of the ereferrals.bcbsm.com website: Our updated training resources Based on provider feedback, we updated the webinar recording and the PDF of the webinar presentation. These updated resources are available on our dedicated provider training site. For information about accessing this site, see the webpages in the links above. More about new and updated documents We’ve added these documents:
We’ve clarified and added information in these documents:
Billing change for IOP services provided via telehealth for some members, for dates of service on or after Oct. 1For dates of service on or after Oct. 1, 2021, you should no longer include procedure code Q3014 on claims for behavioral health intensive outpatient program services provided via telemedicine. Instead, you should bill revenue code 0905 or 0906 with modifier GT or 95. As you may recall, we started allowing IOP via telehealth as a temporary measure in the early days of the COVID-19 pandemic. This change affects all Blue Care Network commercial members, all BCN Advantage℠ members and select Blue Cross Blue Shield of Michigan commercial group members. Keep the following in mind:
We’ve updated the following documents to reflect this change:
You can find these documents on our public website at bcbsm.com/coronavirus or within Provider Secured Services. Note: For Medicare Plus Blue℠ members, see the Medicare-covered telehealth services for the COVID-19 PHE document to determine which IOP and partial hospital program procedure codes are billable for telehealth.
Blue Cross launches 2 new CQIs: MIBAC, MCT2DYou may have first read about these new Collaborative Quality Initiatives in the September-October issue of Hospital and Physician Update. The following article contains some updated statistics and other information. Blue Cross Blue Shield of Michigan has launched two new Collaborative Quality Initiatives — the Michigan Back Collaborative, or MIBAC, and the Michigan Collaborative for Type 2 Diabetes, or MCT2D. These CQIs are expected to improve outcomes for two widespread conditions — low back pain and Type 2 diabetes. This brings the total number of CQIs in our program portfolio to 19. Faris Ahmad, M.D., medical director with Value Partnerships, believes these newest CQIs will make a big difference in the health and well-being of residents throughout Michigan. “The CQI portfolio is of great interest to Blue Cross and a source of pride,” Ahmad said. “Working together with our hospital and physician partners, we continue to find new avenues to explore that can positively affect the outcomes of care for many different areas.” Michigan Back Collaborative Low back pain is one of the most common and expensive conditions for which patients seek care. Currently, care pathways for treating low back pain vary widely. They’re often inefficient and costly, resulting in unsatisfactory outcomes for patients. Many patients don’t improve with either conservative therapy (exercise, medication, physical therapy) or more expensive forms of treatment (injections, surgery). Patients whose acute back pain isn’t effectively managed may begin to experience chronic low back pain, which is associated with disability and potential opioid dependence. That’s one reason effective treatment of patients in the acute phase is crucial. “We commonly see unnecessary imaging studies and referrals, use of unproven therapies, overuse of opioids and lack of effective pain relief,” Ahmad said. MIBAC is using a spine care pathway developed in part by Excellus BlueCross BlueShield in New York, which has been proven effective, resulting in significant improvement in care patterns, better outcomes and lower costs for patients with low back pain. The pathway is helping the CQI accomplish one of its main goals — identifying the cause of low back pain in “first contact” settings to offer less invasive treatment and help avoid unnecessary referrals and imaging. Participation in MIBAC currently includes primary care doctors (M.D.s and D.O.s) affiliated with physician organizations that participate in the Physician Group Incentive Program, along with chiropractors, regardless of PGIP participation. Eventually, the program will be opened to other practitioners involved in treating low back pain. “The Michigan Association of Chiropractors has engaged their providers around this CQI, and we’ve received a good response from primary care doctors as well,” Ahmad said. “We anticipate that by reducing exposure to unnecessary imaging and treatment, we can improve outcomes and the member experience.” The CQI has three levels of participation, the first being online training for primary care physicians and chiropractors. Once trained, providers may then participate in the subsequent levels, which involve data collection and quality initiatives. The collaborative held introductory webinars in February and March, with online training beginning in March. To date, more than 550 primary care physicians and chiropractors have completed the online training. Participation in the advanced levels is scheduled to begin late this year. Henry Ford Medical Group is leading the MIBAC CQI and serving as the coordinating center. Its role includes:
Michigan Collaborative for Type 2 Diabetes Type 2 diabetes is one of the most prevalent, costly and disabling diseases in the U.S. According to the Centers for Disease Control and Prevention, approximately 10% of adults, or about 778,000 Michigan residents, report having a diabetes diagnosis. The data suggests that about 20% of those who have Type 2 diabetes don’t even know it. Adding these undiagnosed cases to the total brings the number of adults in Michigan with Type 2 diabetes closer to 1 million. This results in health care costs of $327 billion in the U.S., or $1 of every $7 spent on health care, with Michigan costs totaling approximately $7.2 billion. In addition, diabetes can lead to kidney failure, heart disease, stroke and peripheral vascular disease. “Approximately 1,000 Michiganders are diagnosed with Type 2 diabetes every week,” Ahmad said. “That’s 52,000 newly diagnosed every year. Statistics like these underscore the urgency of implementing a CQI to address the issue of diabetes.” Currently, there are 25 physician organizations and more than 700 primary care physicians participating in MCT2D. The first meeting of Blue Cross and participants took place Sept. 24. The goal of MCT2D is to prevent, slow and reverse disease progression of Type 2 diabetes. Rather than simply focusing on controlling blood sugar, MCT2D will work to support providers in their efforts to deliver high-quality behavioral and medical treatment to prevent, slow or reverse the course of the disease. To help accomplish this, the collaborative is employing three newly emerging, evidence-based strategies that can lead to improved outcomes for patients with Type 2 diabetes, including reversal of the disease:
“The use of CGM devices promotes patient engagement, provides real-time feedback, and costs less than many other interventions,” Ahmad noted. Michigan Medicine is leading the MCT2D CQI and serving as the coordinating center. The CQI is currently partnering with Physician Group Incentive Program physician organizations to recruit primary care doctors and specialists (endocrinologists and nephrologists) who have a significant number of patients with Type 2 diabetes. MCT2D will be working closing with our POs to roll out this effort and engage providers, using the CQI model of collaboration, data reporting and sharing of best practices. For more information about MIBAC or MCT2D, email CQIprograms@bcbsm.com. For more information about Value Partnerships and other CQIs, visit valuepartnerships.com.
Medicare Plus Blue claims submission process has changed for musculoskeletal procedures that originate in emergency departmentFor claims submitted on or after Oct. 1, 2021, you no longer need to call Provider Inquiry for musculoskeletal surgical and related procedures originating in the emergency department for Medicare Plus Blue℠ members. Instead, you’ll simply submit the claim with an emergency indicator of Y on the CMS-1500 claim form or the SV109 field of the 837P claim transaction. For Blue Cross Blue Shield of Michigan and Blue Care Network commercial and BCN Advantage℠ members, continue to follow your usual process for submitting these claims. As a reminder, you don’t need to request prior authorization from TurningPoint Healthcare Solutions LLC for orthopedic, pain management and spinal procedures when they’re performed emergently during an inpatient admission that originated in the emergency department. To learn more about claims for musculoskeletal procedures, see the “Claims” section of the document titled Musculoskeletal procedure authorizations: Frequently asked questions for providers.
Nonclinical, transitional care program aims to reduce readmissions for Medicare Advantage membersBlue Cross Blue Shield of Michigan and Blue Care Network are contracting with naviHealth to reduce avoidable inpatient readmissions through a nonclinical, transitional care program. This program will be available to Medicare Plus Blue℠ and BCN Advantage℠ members who are discharged from inpatient facilities in Michigan, and will be implemented in two phases:
naviHealth staff will support these members as they transition out of inpatient facilities. These efforts will extend for up to 30 days after members are discharged. With each interaction, naviHealth staff members will introduce themselves to the member, using their name and licensure (if applicable) and the naviHealth name. Prior to discharge from an inpatient facility naviHealth navigation specialists will work with members prior to discharge from an inpatient facility to:
The navigation specialists will share this information with the naviHealth patient navigator who is assigned to the member for post-discharge care. After members leave inpatient facilities naviHealth patient navigators will work with members after discharge from inpatient facilities to:
If the patient navigator has concerns about a member, he or she may reach out to the member’s provider. Note: Patient navigators are commonly known as community health workers. These naviHealth staff members are trusted, knowledgeable frontline personnel who typically reside in or near the communities they serve. Additional information For more information about this program, see the Readmissions Reduction page on naviHealth’s website.** **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
We’re adding requirements for 4 drugs covered under medical benefitFor dates of service on or after Dec. 27, 2021, we’re adding requirements when the following drugs are administered in an outpatient setting for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans:
How to submit authorization requests Submit prior authorization requests through NovoLogix for these drugs. It offers real-time status checks and immediate approvals for certain medications. To learn how to submit requests through NovoLogix, visit the Medical Benefit Drugs page on the ereferrals.bcbsom.com website. Scroll to the Blue Cross commercial column and review the information in the How to submit authorization requests electronically using NovoLogix section. As a reminder, authorization isn’t a guarantee of payment. Health care practitioners should always verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans, see:
We’ll update the appropriate drug lists to reflect the information in this article before the effective date. Note: For other medical benefit drugs, Accredo manages prior authorization requests.
Starting Jan. 1, 2022, we’ll change how we cover some drugsAs we’ve let you know before, Blue Cross Blue Shield of Michigan and Blue Care Network want to make sure members receive safe, high-quality care that meets their needs. To accomplish this, we’re making some changes to how we cover some drugs on the Clinical, Custom, Custom Select and Preferred Drug lists, starting Jan. 1, 2022. We’ll send letters to affected members and their groups and providers. The following is a list of these changes: Drugs on the Clinical and Custom Drug Lists that won’t be covered We’ll no longer cover the drugs in the table below. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after Jan. 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed, along with the covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
1Authorized brand alternatives (authorized generics) are drugs that are considered brand-name drugs and don’t have generic equivalents. These drugs are the same as the brand-name drugs but aren’t true generic drugs. The respective brand copayment ill apply for these drugs. Drugs on Custom Drug List that will have a higher copayment The following brand-name drugs will have a higher copayment, starting Jan. 1, 2022. We’ve listed each along with the preferred alternatives that have similar effectiveness, quality and safety, but lower copays. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
Drugs on the Custom Select Drug List that won’t be covered We’ll no longer cover the following brand-name and generic drugs. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after Jan. 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed along with the covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions for preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
1Authorized brand alternatives (authorized generics) are drugs that are considered brand-name drugs and don’t have generic equivalents. These drugs are the same as the brand-name drugs but aren’t true generic drugs. The respective brand copayment ill apply for these drugs. Drugs on the Custom Select Drug List that will have a higher copayment The following brand-name drugs will have a higher copayment, starting Jan. 1, 2022. We’ve listed each along with the preferred alternatives that have similar effectiveness, quality and safety, but lower copays. When pharmacies fill prescriptions for preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
Drugs on the Preferred Drug List that won’t be covered We’ll no longer cover the following drugs. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after Jan. 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed along with the covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
1Authorized brand alternatives (authorized generics) are drugs that are considered brand-name drugs and don’t have generic equivalents. These drugs are the same as the brand-name drugs but aren’t true generic drugs. The respective brand cost share will apply for these drugs. Drugs on the Preferred Drug List that will have a higher copayment The following brand-name drugs will have a higher copayment, starting Jan. 1, 2022. We’ve listed each along with the preferred alternatives that have similar effectiveness, quality and safety, but lower copays. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
**A closed prescription drug benefit doesn’t cover non-preferred brand drugs. Only generic and preferred brand drugs are covered.
Quarterly update: Requirements changed for some commercial medical benefit drugsBlue Cross Blue Shield of Michigan and Blue Care Network encourage proper utilization of high-cost medications that are covered under the medical benefit. As part of this effort, we maintain a comprehensive list of requirements for Blue Cross and BCN group and individual commercial members. During July, August and September 2021, there were changes to prior authorization requirements, site-of-care requirements or both for Blue Cross and BCN commercial members for the following medical benefit drugs:
Note: The codes shown above will become unique codes. For a detailed list of requirements, see the Blue Cross and BCN utilization management medical drug list. This list is available on the following pages of the ereferrals.bcbsm.com website: As a reminder, an authorization approval isn’t a guarantee of payment. Health care providers need to verify eligibility and benefits for members. Additional information For Blue Cross commercial groups, this authorization requirement applies only to groups that currently participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. A link to this list is also available on the Blue Cross Medical Benefit Drugs page of the ereferrals.bcbsm.com website. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don't participate in the standard prior authorization program.
Ivermectin prescriptions require prior authorization for Blue Cross, BCN commercial membersStarting Oct. 1, 2021, Blue Cross Blue Shield of Michigan and Blue Care Network began temporarily requiring prior authorization for prescriptions of Stromectol®, or ivermectin, for members with commercial coverage. This new rule is effective until further notice. Prior authorization will be granted for indications approved by the U.S. Food and Drug Administration. The quantity of ivermectin tablets dispensed will be limited to 20 tablets per year for commercial members with plans that include quantity limits. The quantity limit is applied over 365 days, not a calendar year. Blue Cross and BCN are adding these requirements to discourage unauthorized use of this medication, such as for treatment of COVID-19. For more information, view the CDC Health Advisory.** Prescriptions for members with Medicare Plus Blue℠ and BCN Advantage℠ coverage aren’t affected. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
New on‑demand training availableProvider Experience continues to offer training resources for health care providers and staff. Our provider training site is available to enhance the training experience for health care providers and staff. And our on-demand courses are designed to help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network. Here are some of the newest resources that are available:
To request access to our training site, follow these steps:
If you need assistance creating your login ID or navigating the site, contact ProviderTraining@bcbsm.com. **RBCE stands for risk-bearing contract entity.
Our e‑learning video series can help you create positive patient experiences and boost Medicare Star RatingsAs you read in the October Record, our e-learning videos — designed for office staff responsible for closing gaps related to Medicare Star measures — launched Aug. 15. The video series addresses the importance of creating positive patient experiences as part of your efforts to close gaps in care. Topics include:
The video series has been approved for AMA PRA Category 1 Credit™. It’s available on our provider training site.
Log in to access the module in the course catalog under Quality management or by entering “Star” in the search box at the top of the screen. Watch this video to learn more about the provider training site. If you need assistance creating your login ID or navigating the site, email ProviderTraining@bcbsm.com. HEDIS®, which stands for Healthcare Effectiveness Data Information Set, is a registered trademark of the National Committee for Quality Assurance.
Provider training website offers e‑learning on E/M guidelines, scenariosWe encourage you to check out our e-learning lesson about evaluation and management guidelines and scenarios. And, as you prepare to submit claims, you can follow the new E/M guidelines outlined in the lesson. The course, available on our provider training site, includes a video summary of key points, along with links to supporting documents from Blue Cross Blue Shield of Michigan. Access to the site will differ slightly for new and existing users.
Once logged in, users have two options for accessing the module:
If you need assistance creating your login ID or navigating the site, email ProviderTraining@bcbsm.com.
There are 2 upcoming lunchtime webinars for physicians and codersAction item We’re offering additional webinars that provide updated information on risk adjustment documentation and coding of common challenging diagnoses. All sessions start at 12:15 p.m. Eastern time and run for 15 to 30 minutes. They also provide physicians and coders with an opportunity to ask questions. Click on a link below to sign up for a live webinar:
You can watch previously hosted sessions on our new provider training site:
Access to the training site differs slightly for new and existing users:
Once logged in, users can access the modules in two ways:
More information
Additional drugs require prior authorization for URMBT members with Blue Cross non‑Medicare plansWe’re adding prior authorization requirements through AIM Specialty Health® for the following drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans. These drugs will require prior authorization for dates of service on or after Dec. 3:
These drugs will require prior authorization for dates of service on or after Jan. 3, 2022:
Prior authorization requirements apply when these drugs are administered in an outpatient setting. This requirement doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714). How to submit authorization requests Submit prior authorization requests to AIM using one of the following methods:
Additional information Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, see:
We’ll update the appropriate drug lists to reflect the information in this article before the effective date. Accredo manages prior authorization requests for additional medical benefit drugs. **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Reminder: Additional edits coming in December for Blue Cross commercial claimsAt Blue Cross Blue Shield of Michigan, we remain committed to payment integrity solutions that support payment accuracy and encourage correct coding. In support of that commitment, you’ll begin to see new edits starting in December. In the August 2021 Record, we let you know about edits that will occur through our new partnership with Optum. The Optum program has two components related to pre-payment edits and medical record requests. You’ll be able to recognize the edits by the unique provider message codes (K500-K544). In some instances, you may receive a letter requesting medical records directly from Optum prior to claim payment. We expect these requests to occur for no more that 1% of claims. Here are some answers to frequently asked questions about medical records requests that may be helpful: How does Optum decide which claims require medical record review? Optum develops customized analytics that are used to identify claims that need additional review. These analytic concepts are developed using Blue Cross Blue Shield of Michigan’s internal payment policies and policies from external agencies, such as the Centers for Medicare & Medicaid Services, as applicable. How do I submit my medical records and what should I include? The Optum medical record request letters will be sent within two business days of a claim being selected for review (referred to as tagging). The request letters will provide detailed instructions of how and where to submit your medical records and what to include with your submission. This includes:
Medical records must be submitted within 60 calendar days of the request. Once received, records will be reviewed within 12 business days and an outcome letter will be sent to you. If no records are received within 60 days, a technical denial letter will be sent as final communication, and Blue Cross will be notified that Optum has closed the case. When the program starts, whom do I contact at Optum for assistance with medical record submission? If you need assistance with submitting your medical records or have any questions, you’ll be able to contact Optum directly at the phone number provided in the medical record request letter. What options do I have if I don’t agree with a denial? When Optum sends its initial findings denial letter, it will include information required if you request a reconsideration of the review. Your information should include:
As a reminder, Optum will conduct its review and send a resolution letter within 12 business days from date of receipt. Timely filing rules will apply.
Pneumatic Compression Device payment policy updateIn support of correct coding and payment accuracy, please be aware Blue Cross Blue Shield of Michigan will be updating its payment policy for pneumatic compressor devices. Beginning in February 2022, we’ll review HCPCS codes E0650-E0651 and E0655-E0673 to make sure claims contain diagnosis codes that are appropriate for the service billed. This policy aligns with the Centers for Medicare & Medicaid Services. According to CMS, the application of pneumatic compression devices is indicated for the treatment of lymphedema or for the treatment of chronic venous insufficiency with venous stasis ulcers. You’ll want to ensure that submitted claims reflect the services performed and the patient’s condition.
SilverSneakers fitness program starts in January for MPSERS Medicare Plus Blue membersThe SilverSneakers® fitness program will be available at no additional cost, starting Jan. 1, 2022, for all Michigan Public School Employees' Retirement System members with Medicare Plus Blue℠ plans. SilverSneakers is the nation's leading exercise program designed to keep older adults active and healthy. It offers exercise and nutrition classes at participating fitness centers and online, either live or on-demand.The classes consist of no-impact cardiovascular and muscular strength activities that have been found to be the most helpful for seniors. The fitness program encourages members to live healthier, more active lifestyles, and can help prevent many of the problems that typically occur with aging. Increasing physical activity helps strengthen bones and improve joint flexibility, and makes seniors feel safer when performing their daily activities. The program also provides members with information about weight control and explores exercise options that prove helpful for older adults with chronic conditions such as high blood pressure, diabetes, obesity and osteoarthritis. The SilverSneakers Fitness Program is a great resource for your eligible senior patients. Visit silversneakers.com** to learn more. **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
We’ve created a diabetes toolkit to help membersIn recognition of National Diabetes Month in November, Blue Cross Blue Shield of Michigan has developed a diabetes flyer — part of a toolkit of materials that employers can use with their employees to help keep them healthy. We’re encouraging members who have diabetes to reach out to their providers to stay on top of their diabetes care. We let them know it’s important to make sure they have consistent A1c checkups with their primary care provider and a retinal eye exam with an ophthalmologist or optometrist. The materials in the diabetes toolkit can be used year-round, not just in November. You’re welcome to use the toolkit — or share the flyer — with patients in your practice. Did you know? According to the National Diabetes Statistics Report 2020,** diabetes is the leading cause of new vision loss. So we encourage you to talk with your patients about the importance of a retinal eye exam and how it fits into their diabetes management plan. **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Help your patients with medication adherenceThis is part of an ongoing series of articles focusing on the tools and resources available to help FEP® members manage their health. An estimated 50% of patients prescribed a medication don’t take it as ordered, according to the American Medical Association. When patients adhere to their medication plan, it reduces the patient’s overall health care costs by helping them avoid adverse events — particularly important for patients with a chronic condition. However, as you know, helping patients who have asthma, diabetes, hypertension, high cholesterol and other chronic conditions adhere to their medication regimen can be a challenge. An AMA STEPS Forward™ module** on medication adherence was developed in 2015 to help physicians improve their patient’s adherence. The module lists eight steps to help improve medication adherence:
An AMA article, “8 reasons patients don’t take their medications,”** further discusses the reasons for medication nonadherence. The Blue Cross and Blue Shield Federal Employee Program® Service Benefit Plan supports members with resources for medication adherence, including:
If you or a member has questions about support services and benefits, call Customer Service at 1-800-482-3600. **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
CareCentrix home health care program: Updated training resources, new and updated documents availableCareCentrix® manages prior authorizations for home health care services for Medicare Plus Blue℠ and BCN Advantage℠ members as follows:
Where to find CareCentrix home health care resources You can find the training resources and links to the documents related to this program on the following pages of the ereferrals.bcbsm.com website: Our updated training resources Based on provider feedback, we updated the webinar recording and the PDF of the webinar presentation. These updated resources are available on our dedicated provider training site. For information about accessing this site, see the webpages in the links above. More about new and updated documents We’ve added these documents:
We’ve clarified and added information in these documents:
Billing change for IOP services provided via telehealth for some members, for dates of service on or after Oct. 1For dates of service on or after Oct. 1, 2021, you should no longer include procedure code Q3014 on claims for behavioral health intensive outpatient program services provided via telemedicine. Instead, you should bill revenue code 0905 or 0906 with modifier GT or 95. As you may recall, we started allowing IOP via telehealth as a temporary measure in the early days of the COVID-19 pandemic. This change affects all Blue Care Network commercial members, all BCN Advantage℠ members and select Blue Cross Blue Shield of Michigan commercial group members. Keep the following in mind:
We’ve updated the following documents to reflect this change:
You can find these documents on our public website at bcbsm.com/coronavirus or within Provider Secured Services. Note: For Medicare Plus Blue℠ members, see the Medicare-covered telehealth services for the COVID-19 PHE document to determine which IOP and partial hospital program procedure codes are billable for telehealth.
Lessons learned from Mi‑COVID19 InitiativeWhat you need to know The Mi‑COVID19 initiative, a joint effort among several Blue Cross Blue Shield of Michigan Collaborative Quality Initiatives, has played a key role in Michigan’s response to the COVID‑19 pandemic. “This has been a journey that we have taken together over the last year,” said Scott Flanders, M.D., the initiative’s program director and chief clinical strategy officer at Michigan Medicine. “A large group of health professionals shared crucial information, which significantly improved the outcomes of our patients here in Michigan.” Data collection and sharing By the end of January 2021, data on more than 3,500 patients with COVID‑19 had been abstracted, analyzed and shared. In addition to sharing best practices in more than 30 webinars, four peer-reviewed papers have been published, with another seven currently under review. Key insights In June 2021, the Mi‑COVID19 registry leaders presented a webinar to Michigan hospitals, physicians and other health care leaders on the lessons learned during the COVID‑19 pandemic. They shared the following key insights:
In addition, initiative leaders have developed a mortality risk assessment model,** which enables providers to assess a patient’s risk of death at the time of admission. This allows providers to use appropriate treatment protocols more quickly. “All of us can be proud of the important work we were able to accomplish in coordinating resources to best treat COVID‑19,” said Amy McKenzie, M.D., associate chief medical officer at Blue Cross and one of the Mi‑COVID19 CQI steering committee members. “We were able to get the CQI up and running and collecting data within a month and determine notable variations in care and arrive at best practices within a relatively short period of time. All this helped provide our hospitalized COVID patients across Michigan access to cutting-edge care.” More information on the Mi‑COVID19 initiative can be found on the Michigan Hospital Medicine Safety Consortium website.** **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Blue Cross launches 2 new CQIs: MIBAC, MCT2DYou may have first read about these new Collaborative Quality Initiatives in the September-October issue of Hospital and Physician Update. The following article contains some updated statistics and other information. Blue Cross Blue Shield of Michigan has launched two new Collaborative Quality Initiatives — the Michigan Back Collaborative, or MIBAC, and the Michigan Collaborative for Type 2 Diabetes, or MCT2D. These CQIs are expected to improve outcomes for two widespread conditions — low back pain and Type 2 diabetes. This brings the total number of CQIs in our program portfolio to 19. Faris Ahmad, M.D., medical director with Value Partnerships, believes these newest CQIs will make a big difference in the health and well-being of residents throughout Michigan. “The CQI portfolio is of great interest to Blue Cross and a source of pride,” Ahmad said. “Working together with our hospital and physician partners, we continue to find new avenues to explore that can positively affect the outcomes of care for many different areas.” Michigan Back Collaborative Low back pain is one of the most common and expensive conditions for which patients seek care. Currently, care pathways for treating low back pain vary widely. They’re often inefficient and costly, resulting in unsatisfactory outcomes for patients. Many patients don’t improve with either conservative therapy (exercise, medication, physical therapy) or more expensive forms of treatment (injections, surgery). Patients whose acute back pain isn’t effectively managed may begin to experience chronic low back pain, which is associated with disability and potential opioid dependence. That’s one reason effective treatment of patients in the acute phase is crucial. “We commonly see unnecessary imaging studies and referrals, use of unproven therapies, overuse of opioids and lack of effective pain relief,” Ahmad said. MIBAC is using a spine care pathway developed in part by Excellus BlueCross BlueShield in New York, which has been proven effective, resulting in significant improvement in care patterns, better outcomes and lower costs for patients with low back pain. The pathway is helping the CQI accomplish one of its main goals — identifying the cause of low back pain in “first contact” settings to offer less invasive treatment and help avoid unnecessary referrals and imaging. Participation in MIBAC currently includes primary care doctors (M.D.s and D.O.s) affiliated with physician organizations that participate in the Physician Group Incentive Program, along with chiropractors, regardless of PGIP participation. Eventually, the program will be opened to other practitioners involved in treating low back pain. “The Michigan Association of Chiropractors has engaged their providers around this CQI, and we’ve received a good response from primary care doctors as well,” Ahmad said. “We anticipate that by reducing exposure to unnecessary imaging and treatment, we can improve outcomes and the member experience.” The CQI has three levels of participation, the first being online training for primary care physicians and chiropractors. Once trained, providers may then participate in the subsequent levels, which involve data collection and quality initiatives. The collaborative held introductory webinars in February and March, with online training beginning in March. To date, more than 550 primary care physicians and chiropractors have completed the online training. Participation in the advanced levels is scheduled to begin late this year. Henry Ford Medical Group is leading the MIBAC CQI and serving as the coordinating center. Its role includes:
Michigan Collaborative for Type 2 Diabetes Type 2 diabetes is one of the most prevalent, costly and disabling diseases in the U.S. According to the Centers for Disease Control and Prevention, approximately 10% of adults, or about 778,000 Michigan residents, report having a diabetes diagnosis. The data suggests that about 20% of those who have Type 2 diabetes don’t even know it. Adding these undiagnosed cases to the total brings the number of adults in Michigan with Type 2 diabetes closer to 1 million. This results in health care costs of $327 billion in the U.S., or $1 of every $7 spent on health care, with Michigan costs totaling approximately $7.2 billion. In addition, diabetes can lead to kidney failure, heart disease, stroke and peripheral vascular disease. “Approximately 1,000 Michiganders are diagnosed with Type 2 diabetes every week,” Ahmad said. “That’s 52,000 newly diagnosed every year. Statistics like these underscore the urgency of implementing a CQI to address the issue of diabetes.” Currently, there are 25 physician organizations and more than 700 primary care physicians participating in MCT2D. The first meeting of Blue Cross and participants took place Sept. 24. The goal of MCT2D is to prevent, slow and reverse disease progression of Type 2 diabetes. Rather than simply focusing on controlling blood sugar, MCT2D will work to support providers in their efforts to deliver high-quality behavioral and medical treatment to prevent, slow or reverse the course of the disease. To help accomplish this, the collaborative is employing three newly emerging, evidence-based strategies that can lead to improved outcomes for patients with Type 2 diabetes, including reversal of the disease:
“The use of CGM devices promotes patient engagement, provides real-time feedback, and costs less than many other interventions,” Ahmad noted. Michigan Medicine is leading the MCT2D CQI and serving as the coordinating center. The CQI is currently partnering with Physician Group Incentive Program physician organizations to recruit primary care doctors and specialists (endocrinologists and nephrologists) who have a significant number of patients with Type 2 diabetes. MCT2D will be working closing with our POs to roll out this effort and engage providers, using the CQI model of collaboration, data reporting and sharing of best practices. For more information about MIBAC or MCT2D, email CQIprograms@bcbsm.com. For more information about Value Partnerships and other CQIs, visit valuepartnerships.com.
Medicare Plus Blue claims submission process has changed for musculoskeletal procedures that originate in emergency departmentFor claims submitted on or after Oct. 1, 2021, you no longer need to call Provider Inquiry for musculoskeletal surgical and related procedures originating in the emergency department for Medicare Plus Blue℠ members. Instead, you’ll simply submit the claim with an emergency indicator of Y on the CMS-1500 claim form or the SV109 field of the 837P claim transaction. For Blue Cross Blue Shield of Michigan and Blue Care Network commercial and BCN Advantage℠ members, continue to follow your usual process for submitting these claims. As a reminder, you don’t need to request prior authorization from TurningPoint Healthcare Solutions LLC for orthopedic, pain management and spinal procedures when they’re performed emergently during an inpatient admission that originated in the emergency department. To learn more about claims for musculoskeletal procedures, see the “Claims” section of the document titled Musculoskeletal procedure authorizations: Frequently asked questions for providers.
Nonclinical, transitional care program aims to reduce readmissions for Medicare Advantage membersBlue Cross Blue Shield of Michigan and Blue Care Network are contracting with naviHealth to reduce avoidable inpatient readmissions through a nonclinical, transitional care program. This program will be available to Medicare Plus Blue℠ and BCN Advantage℠ members who are discharged from inpatient facilities in Michigan, and will be implemented in two phases:
naviHealth staff will support these members as they transition out of inpatient facilities. These efforts will extend for up to 30 days after members are discharged. With each interaction, naviHealth staff members will introduce themselves to the member, using their name and licensure (if applicable) and the naviHealth name. Prior to discharge from an inpatient facility naviHealth navigation specialists will work with members prior to discharge from an inpatient facility to:
The navigation specialists will share this information with the naviHealth patient navigator who is assigned to the member for post-discharge care. After members leave inpatient facilities naviHealth patient navigators will work with members after discharge from inpatient facilities to:
If the patient navigator has concerns about a member, he or she may reach out to the member’s provider. Note: Patient navigators are commonly known as community health workers. These naviHealth staff members are trusted, knowledgeable frontline personnel who typically reside in or near the communities they serve. Additional information For more information about this program, see the Readmissions Reduction page on naviHealth’s website.** **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
We’re adding requirements for 4 drugs covered under medical benefitFor dates of service on or after Dec. 27, 2021, we’re adding requirements when the following drugs are administered in an outpatient setting for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans:
How to submit authorization requests Submit prior authorization requests through NovoLogix for these drugs. It offers real-time status checks and immediate approvals for certain medications. To learn how to submit requests through NovoLogix, visit the Medical Benefit Drugs page on the ereferrals.bcbsom.com website. Scroll to the Blue Cross commercial column and review the information in the How to submit authorization requests electronically using NovoLogix section. As a reminder, authorization isn’t a guarantee of payment. Health care practitioners should always verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans, see:
We’ll update the appropriate drug lists to reflect the information in this article before the effective date. Note: For other medical benefit drugs, Accredo manages prior authorization requests.
Starting Jan. 1, 2022, we’ll change how we cover some drugsAs we’ve let you know before, Blue Cross Blue Shield of Michigan and Blue Care Network want to make sure members receive safe, high-quality care that meets their needs. To accomplish this, we’re making some changes to how we cover some drugs on the Clinical, Custom, Custom Select and Preferred Drug lists, starting Jan. 1, 2022. We’ll send letters to affected members and their groups and providers. The following is a list of these changes: Drugs on the Clinical and Custom Drug Lists that won’t be covered We’ll no longer cover the drugs in the table below. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after Jan. 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed, along with the covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
1Authorized brand alternatives (authorized generics) are drugs that are considered brand-name drugs and don’t have generic equivalents. These drugs are the same as the brand-name drugs but aren’t true generic drugs. The respective brand copayment ill apply for these drugs. Drugs on Custom Drug List that will have a higher copayment The following brand-name drugs will have a higher copayment, starting Jan. 1, 2022. We’ve listed each along with the preferred alternatives that have similar effectiveness, quality and safety, but lower copays. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
Drugs on the Custom Select Drug List that won’t be covered We’ll no longer cover the following brand-name and generic drugs. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after Jan. 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed along with the covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions for preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
1Authorized brand alternatives (authorized generics) are drugs that are considered brand-name drugs and don’t have generic equivalents. These drugs are the same as the brand-name drugs but aren’t true generic drugs. The respective brand copayment ill apply for these drugs. Drugs on the Custom Select Drug List that will have a higher copayment The following brand-name drugs will have a higher copayment, starting Jan. 1, 2022. We’ve listed each along with the preferred alternatives that have similar effectiveness, quality and safety, but lower copays. When pharmacies fill prescriptions for preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
Drugs on the Preferred Drug List that won’t be covered We’ll no longer cover the following drugs. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after Jan. 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed along with the covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
1Authorized brand alternatives (authorized generics) are drugs that are considered brand-name drugs and don’t have generic equivalents. These drugs are the same as the brand-name drugs but aren’t true generic drugs. The respective brand cost share will apply for these drugs. Drugs on the Preferred Drug List that will have a higher copayment The following brand-name drugs will have a higher copayment, starting Jan. 1, 2022. We’ve listed each along with the preferred alternatives that have similar effectiveness, quality and safety, but lower copays. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
**A closed prescription drug benefit doesn’t cover non-preferred brand drugs. Only generic and preferred brand drugs are covered.
Quarterly update: Requirements changed for some commercial medical benefit drugsBlue Cross Blue Shield of Michigan and Blue Care Network encourage proper utilization of high-cost medications that are covered under the medical benefit. As part of this effort, we maintain a comprehensive list of requirements for Blue Cross and BCN group and individual commercial members. During July, August and September 2021, there were changes to prior authorization requirements, site-of-care requirements or both for Blue Cross and BCN commercial members for the following medical benefit drugs:
Note: The codes shown above will become unique codes. For a detailed list of requirements, see the Blue Cross and BCN utilization management medical drug list. This list is available on the following pages of the ereferrals.bcbsm.com website: As a reminder, an authorization approval isn’t a guarantee of payment. Health care providers need to verify eligibility and benefits for members. Additional information For Blue Cross commercial groups, this authorization requirement applies only to groups that currently participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. A link to this list is also available on the Blue Cross Medical Benefit Drugs page of the ereferrals.bcbsm.com website. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don't participate in the standard prior authorization program.
Ivermectin prescriptions require prior authorization for Blue Cross, BCN commercial membersStarting Oct. 1, 2021, Blue Cross Blue Shield of Michigan and Blue Care Network began temporarily requiring prior authorization for prescriptions of Stromectol®, or ivermectin, for members with commercial coverage. This new rule is effective until further notice. Prior authorization will be granted for indications approved by the U.S. Food and Drug Administration. The quantity of ivermectin tablets dispensed will be limited to 20 tablets per year for commercial members with plans that include quantity limits. The quantity limit is applied over 365 days, not a calendar year. Blue Cross and BCN are adding these requirements to discourage unauthorized use of this medication, such as for treatment of COVID-19. For more information, view the CDC Health Advisory.** Prescriptions for members with Medicare Plus Blue℠ and BCN Advantage℠ coverage aren’t affected. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
New on‑demand training availableProvider Experience continues to offer training resources for health care providers and staff. Our provider training site is available to enhance the training experience for health care providers and staff. And our on-demand courses are designed to help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network. Here are some of the newest resources that are available:
To request access to our training site, follow these steps:
If you need assistance creating your login ID or navigating the site, contact ProviderTraining@bcbsm.com. **RBCE stands for risk-bearing contract entity.
There are 2 upcoming lunchtime webinars for physicians and codersAction item We’re offering additional webinars that provide updated information on risk adjustment documentation and coding of common challenging diagnoses. All sessions start at 12:15 p.m. Eastern time and run for 15 to 30 minutes. They also provide physicians and coders with an opportunity to ask questions. Click on a link below to sign up for a live webinar:
You can watch previously hosted sessions on our new provider training site:
Access to the training site differs slightly for new and existing users:
Once logged in, users can access the modules in two ways:
More information
Clinical review requirements for admission to skilled nursing facilities from Michigan hospitals suspendedAs we wrote in a web-DENIS message posted Sept. 2, Blue Cross Blue Shield of Michigan and Blue Care Network are temporarily suspending clinical review requirements for the first three days of admission to skilled nursing facilities from all Michigan hospitals due to the latest surge in COVID-19 cases. This temporary change was effective Sept. 20, 2021, and will remain in effect until further notice. It applies to all lines of business, including Blue Cross Blue Shield of Michigan commercial, Blue Care Network commercial, Medicare Plus Blue℠ and BCN Advantage℠. Blue Cross and BCN began suspending clinical review requirements for the first three days of admission to skilled nursing facilities Sept. 1 for states most affected by the surge in COVID-19 cases. For the list of states with this clinical review suspension, refer to this Provider Alert. Information you need to know
The information in this article was valid as of Oct. 14. It’s possible that the suspension may be lifted prior to the publication of the November Record.
Prior authorization requests for commercial SNF admissions will need to be submitted through e‑referral systemBeginning Jan. 1, 2022, we’ll require skilled nursing facilities to submit prior authorization requests through the e-referral system and not by fax. This applies to requests for our Blue Cross Blue Shield of Michigan and Blue Care Network commercial members for:
In September 2020, we encouraged SNF providers to submit commercial prior authorization requests through the e‑referral system starting Dec. 1, 2020. Many SNFs have begun doing that but others are still faxing the requests. What’s changing Starting Jan. 1:
If we receive a faxed form for a non-urgent admission when the e-referral system is available, we won’t accept the request. We’ll notify you by fax or phone that you must submit the request through the e-referral system. Sign up now to use e-referral system To prepare for this change, it’s important that SNFs sign up now for access to the e-referral system. Don’t wait to sign up, as it may take some time to get access. You’ll also need to learn how to use the e-referral system so you’re comfortable with it when this change goes into effect. Everything you need to know is on our ereferrals.bcbsm.com website:
Do’s and don’ts when submitting through the e-referral system For tips on how to make it easier to use the e-referral system when submitting commercial SNF prior authorization requests, refer to the article we published in the May 2021 issue of The Record titled “Do’s and don’ts when submitting commercial SNF requests using the e‑referral system.” Earlier communications about this change In 2020, we communicated about submitting these requests through the e-referral system through these articles:
In these articles, we encouraged SNF providers to sign up for and start using the e‑referral system while faxing was still an option. We also communicated about this through a web-DENIS message and a news item on our ereferrals.bcbsm.com website. As a reminder, naviHealth is an independent company that manages prior authorization requests for SNF admissions for our Medicare Plus Blue℠ and BCN Advantage℠ members.
Facility input requested on SecureCare Network Performance Program implementationBlue Cross Blue Shield of Michigan proposes that the SecureCare Network Performance Management Program be implemented April 1, 2022, for Blue Cross’ commercial and Medicare Plus Blue℠ members. This would replace:
Physical therapists, occupational therapists, speech therapists and chiropractors will be asked to join the SecureCare network. There would be an annual cost to providers estimated at 2% of their Blue Cross commercial claims to join the network. (There’s no additional cost for Medicare Plus Blue claims.) SecureCare allows for these provider types to treat patients without prior authorization or a treatment plan. Other benefits include:
Note: Blue Care Network and BCN Advantage℠ will continue using eviCore. Input requested According to the Contract Administration Process — part of the Participating Hospital Agreement that went into effect July 1, 2021 — we allow non-binding input from participating hospitals about such proposals. Input from facilities is requested by Nov. 30, 2021. Send any input you may have to Liz Bowman at ebowman@bcbsm.com. After input is received, Blue Cross has 30 calendar days to respond to input.
Additional drugs require prior authorization for URMBT members with Blue Cross non‑Medicare plansWe’re adding prior authorization requirements through AIM Specialty Health® for the following drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans. These drugs will require prior authorization for dates of service on or after Dec. 3:
These drugs will require prior authorization for dates of service on or after Jan. 3, 2022:
Prior authorization requirements apply when these drugs are administered in an outpatient setting. This requirement doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714). How to submit authorization requests Submit prior authorization requests to AIM using one of the following methods:
Additional information Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, see:
We’ll update the appropriate drug lists to reflect the information in this article before the effective date. Accredo manages prior authorization requests for additional medical benefit drugs. **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Reminder: Additional edits coming in December for Blue Cross commercial claimsAt Blue Cross Blue Shield of Michigan, we remain committed to payment integrity solutions that support payment accuracy and encourage correct coding. In support of that commitment, you’ll begin to see new edits starting in December. In the August 2021 Record, we let you know about edits that will occur through our new partnership with Optum. The Optum program has two components related to pre-payment edits and medical record requests. You’ll be able to recognize the edits by the unique provider message codes (K500-K544). In some instances, you may receive a letter requesting medical records directly from Optum prior to claim payment. We expect these requests to occur for no more that 1% of claims. Here are some answers to frequently asked questions about medical records requests that may be helpful: How does Optum decide which claims require medical record review? Optum develops customized analytics that are used to identify claims that need additional review. These analytic concepts are developed using Blue Cross Blue Shield of Michigan’s internal payment policies and policies from external agencies, such as the Centers for Medicare & Medicaid Services, as applicable. How do I submit my medical records and what should I include? The Optum medical record request letters will be sent within two business days of a claim being selected for review (referred to as tagging). The request letters will provide detailed instructions of how and where to submit your medical records and what to include with your submission. This includes:
Medical records must be submitted within 60 calendar days of the request. Once received, records will be reviewed within 12 business days and an outcome letter will be sent to you. If no records are received within 60 days, a technical denial letter will be sent as final communication, and Blue Cross will be notified that Optum has closed the case. When the program starts, whom do I contact at Optum for assistance with medical record submission? If you need assistance with submitting your medical records or have any questions, you’ll be able to contact Optum directly at the phone number provided in the medical record request letter. What options do I have if I don’t agree with a denial? When Optum sends its initial findings denial letter, it will include information required if you request a reconsideration of the review. Your information should include:
As a reminder, Optum will conduct its review and send a resolution letter within 12 business days from date of receipt. Timely filing rules will apply.
Supplier partner change for commercial DRG audits begins November 2021EquiClaim, an independent company that provides auditing support for Blue Cross Blue Shield of Michigan and Blue Care Network, will perform claim audits on inpatient hospital diagnosis‑related groups, or DRGs. The audits will:
Medical records will be reviewed to:
You’ll need to provide medical charts for review at the time of an audit. After an audit, EquiClaim will send you a letter with the findings and information on how to request an appeal. Contact the EquiClaim Customer Service Line at 1-866-481-1479 if you have any questions or need to request an extension.
Pneumatic Compression Device payment policy updateIn support of correct coding and payment accuracy, please be aware Blue Cross Blue Shield of Michigan will be updating its payment policy for pneumatic compressor devices. Beginning in February 2022, we’ll review HCPCS codes E0650-E0651 and E0655-E0673 to make sure claims contain diagnosis codes that are appropriate for the service billed. This policy aligns with the Centers for Medicare & Medicaid Services. According to CMS, the application of pneumatic compression devices is indicated for the treatment of lymphedema or for the treatment of chronic venous insufficiency with venous stasis ulcers. You’ll want to ensure that submitted claims reflect the services performed and the patient’s condition.
Starting Jan. 1, 2022, we’ll change how we cover some drugsAs we’ve let you know before, Blue Cross Blue Shield of Michigan and Blue Care Network want to make sure members receive safe, high-quality care that meets their needs. To accomplish this, we’re making some changes to how we cover some drugs on the Clinical, Custom, Custom Select and Preferred Drug lists, starting Jan. 1, 2022. We’ll send letters to affected members and their groups and providers. The following is a list of these changes: Drugs on the Clinical and Custom Drug Lists that won’t be covered We’ll no longer cover the drugs in the table below. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after Jan. 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed, along with the covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
1Authorized brand alternatives (authorized generics) are drugs that are considered brand-name drugs and don’t have generic equivalents. These drugs are the same as the brand-name drugs but aren’t true generic drugs. The respective brand copayment ill apply for these drugs. Drugs on Custom Drug List that will have a higher copayment The following brand-name drugs will have a higher copayment, starting Jan. 1, 2022. We’ve listed each along with the preferred alternatives that have similar effectiveness, quality and safety, but lower copays. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
Drugs on the Custom Select Drug List that won’t be covered We’ll no longer cover the following brand-name and generic drugs. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after Jan. 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed along with the covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions for preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
1Authorized brand alternatives (authorized generics) are drugs that are considered brand-name drugs and don’t have generic equivalents. These drugs are the same as the brand-name drugs but aren’t true generic drugs. The respective brand copayment ill apply for these drugs. Drugs on the Custom Select Drug List that will have a higher copayment The following brand-name drugs will have a higher copayment, starting Jan. 1, 2022. We’ve listed each along with the preferred alternatives that have similar effectiveness, quality and safety, but lower copays. When pharmacies fill prescriptions for preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
Drugs on the Preferred Drug List that won’t be covered We’ll no longer cover the following drugs. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after Jan. 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed along with the covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
1Authorized brand alternatives (authorized generics) are drugs that are considered brand-name drugs and don’t have generic equivalents. These drugs are the same as the brand-name drugs but aren’t true generic drugs. The respective brand cost share will apply for these drugs. Drugs on the Preferred Drug List that will have a higher copayment The following brand-name drugs will have a higher copayment, starting Jan. 1, 2022. We’ve listed each along with the preferred alternatives that have similar effectiveness, quality and safety, but lower copays. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. The example brand names of preferred alternatives are provided for reference. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
**A closed prescription drug benefit doesn’t cover non-preferred brand drugs. Only generic and preferred brand drugs are covered.
Quarterly update: Requirements changed for some commercial medical benefit drugsBlue Cross Blue Shield of Michigan and Blue Care Network encourage proper utilization of high-cost medications that are covered under the medical benefit. As part of this effort, we maintain a comprehensive list of requirements for Blue Cross and BCN group and individual commercial members. During July, August and September 2021, there were changes to prior authorization requirements, site-of-care requirements or both for Blue Cross and BCN commercial members for the following medical benefit drugs:
Note: The codes shown above will become unique codes. For a detailed list of requirements, see the Blue Cross and BCN utilization management medical drug list. This list is available on the following pages of the ereferrals.bcbsm.com website: As a reminder, an authorization approval isn’t a guarantee of payment. Health care providers need to verify eligibility and benefits for members. Additional information For Blue Cross commercial groups, this authorization requirement applies only to groups that currently participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. A link to this list is also available on the Blue Cross Medical Benefit Drugs page of the ereferrals.bcbsm.com website. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don't participate in the standard prior authorization program.
Ivermectin prescriptions require prior authorization for Blue Cross, BCN commercial membersStarting Oct. 1, 2021, Blue Cross Blue Shield of Michigan and Blue Care Network began temporarily requiring prior authorization for prescriptions of Stromectol®, or ivermectin, for members with commercial coverage. This new rule is effective until further notice. Prior authorization will be granted for indications approved by the U.S. Food and Drug Administration. The quantity of ivermectin tablets dispensed will be limited to 20 tablets per year for commercial members with plans that include quantity limits. The quantity limit is applied over 365 days, not a calendar year. Blue Cross and BCN are adding these requirements to discourage unauthorized use of this medication, such as for treatment of COVID-19. For more information, view the CDC Health Advisory.** Prescriptions for members with Medicare Plus Blue℠ and BCN Advantage℠ coverage aren’t affected. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Additional drugs require prior authorization for URMBT members with Blue Cross non‑Medicare plansWe’re adding prior authorization requirements through AIM Specialty Health® for the following drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans. These drugs will require prior authorization for dates of service on or after Dec. 3:
These drugs will require prior authorization for dates of service on or after Jan. 3, 2022:
Prior authorization requirements apply when these drugs are administered in an outpatient setting. This requirement doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714). How to submit authorization requests Submit prior authorization requests to AIM using one of the following methods:
Additional information Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, see:
We’ll update the appropriate drug lists to reflect the information in this article before the effective date. Accredo manages prior authorization requests for additional medical benefit drugs. **Blue Cross Blue Shield of Michigan doesn’t own or control this website. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
No portion of this publication may be copied without the express written permission of Blue Cross Blue Shield of Michigan, except that BCBSM participating health care providers may make copies for their personal use. In no event may any portion of this publication be copied or reprinted and used for commercial purposes by any party other than BCBSM.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||