Subscribe | The Record Archive | Contacts | bcbsm.com
|
April 2021
Blue Cross and BCN extend no‑cost COVID‑19 treatment through Sept. 30, 2021Blue Cross Blue Shield of Michigan and Blue Care Network have extended cost‑share waivers through Sept. 30 for members in Blue Cross and BCN health plans who are diagnosed and treated for COVID‑19. This extension of this temporary benefit, which was set to expire on March 31, ensures members will not pay out‑of‑pocket costs — copays, deductibles, or coinsurance — for the medical care and pharmacy costs associated with COVID-19. You can find additional information on our website at bcbsm.com/coronavirus and our corporate blog at mibluesperspectives.com.
Get ready for Availity: How to designate resources as ‘favorites’
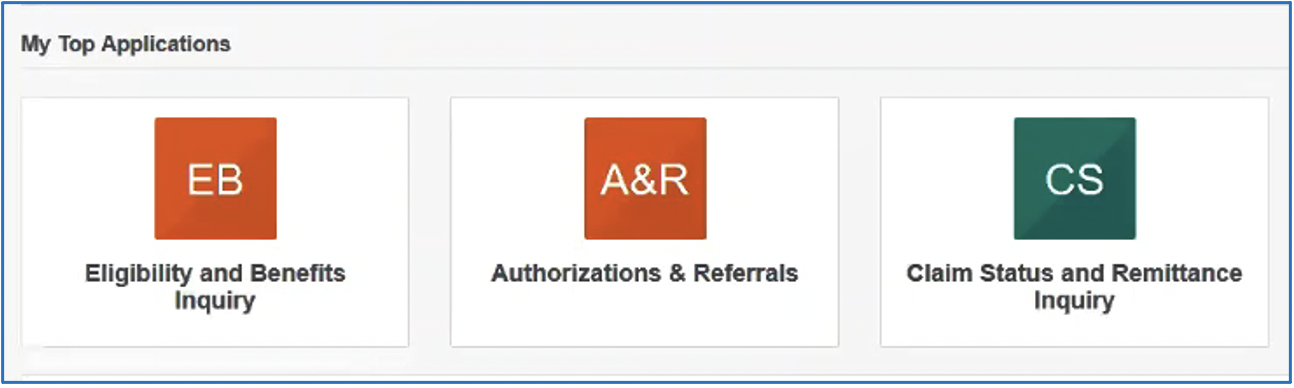
Throughout the Availity portal, you’ll see hearts next to applications and other resources. You can click on the heart if you want to identify that item as one of your favorites. The items you select as favorites are added to the My Favorites drop‑down list at the top of the screen. You can choose what you want shown in that drop‑down list. It can be an application or a specific document. Then, each time you log in to Availity, you can go to My Favorites to quickly find the information you need. In addition, Availity looks at the applications you use the most and lists those on the homepage in the My Top Applications area. Here’s a sample of what you might see based on your usage history.
Questions? If you need immediate assistance or have a question specific to a certain member or situation, use our website resources or contact Provider Inquiry. Web resources:
Provider Inquiry numbers are available at bcbsm.com/providers. Click on Contact Us. Then click on the type of provider you are, and click on Provider Inquiry. Call the Blue Cross Web Support Help Desk at 1‑877‑258‑3932 if you have problems with the current Blue Cross provider portal. Previous articles about Availity
Next Drug Take Back Day scheduled for April 24Remind your patients that next National Prescription Drug Take Back Day is scheduled for April 24 from 10 a.m. to 2 p.m. These twice‑yearly events, coordinated by the U.S. Drug Enforcement Administration, are a key tool in our efforts to battle the opioid epidemic. They provide a safe, convenient and responsible means of disposing of prescription drugs, while educating the public about the potential for abuse of medications. At the most recent Drug Take Back Day in October, about 985,392 pounds of drugs were collected nationwide. As in previous years, Blue Cross Blue Shield of Michigan supports Drug Take Back Day in various ways. For example, we post blogs on MI Blues Perspectives and offer resources to help people responsibly dispose of unused or expired drugs on our Opioids 101 site. To find a drug disposal facility near you that’s participating in Drug Take Back Day, check out the DEA’s search tool** or see Michigan OPEN’s Opioid Disposal Map.** Keep in mind that people who miss the Take Back events don’t need to wait until the next event to safely dispose of unused drugs. For tips on how to safely dispose of unused drugs year‑round, see the May 2018 Record article. As a reminder, Meijer has a Consumer Drug Take‑Back Program** in all Midwest stores. And people can dispose of unused prescription drugs at select Walgreens locations across the state. Read more about Blue Cross’ partnership with Walgreens by clicking here. For more information on disposing of prescription drugs, visit the DEA Diversion Control Division website** or Michigan OPEN’s Opioid Disposal Information and Resources page.** **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
CPT update: New, deleted and updated codesPathology and Laboratory — Proprietary Laboratory Analysis Codes
Surgery — Respiratory System — Nose
You may also want to check out the HCPCS update article in this issue. For information on other codes, you can refer to our 2021 CPT and HCPCS Update document, which was posted on the Clinical Criteria & Resources page of web‑DENIS early this year. It provided information on the new and deleted codes as of Jan. 1, 2021. None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
HCPCS update: New and updated codes
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Code | Change | Coverage comments | Effective date |
|---|---|---|---|
| C9072 | Updated |
Covered for facility only | Jan. 1, 2021 |
| C9073 | Updated |
Covered for facility only | Jan. 1, 2021 |
| G2020 | Added |
Not covered | April 1, 2021 |
| J1823 | Updated |
Covered | Jan. 1, 2021 |
You may also want to check out the CPT update article in this issue.
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
Billing chart: Blues highlight medical, benefit policy changes
You’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart.
This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures.
You will also see that descriptions for the codes are no longer included. This is a result of recent negotiations with the AMA on use of the codes.
We will publish information about new BCBS groups or changes to group benefits under the Group Benefit Changes heading.
For more detailed descriptions of the BCBSM policies for these procedures, please check under the Medical/Payment Policy tab in Explainer on web-DENIS. To access this online information:
- Log in to web-DENIS.
- Click on BCBSM Provider Publications & Resources.
- Click on Benefit Policy for a Code.
- Click on Topic.
- Under Topic Criteria, click on the drop-down arrow next to Choose Identifier Type and then click on HCPCS Code.
- Enter the procedure code.
- Click on Finish.
- Click on Search.
| Code* | BCBSM changes to: Basic Benefit and Medical Policy, Group Variations Payment Policy, Guidelines |
|---|---|
| NEW PAYABLE PROCEDURES | |
90694 |
Basic benefit and medical policy 90694 covered for FDA-approved indications Effective July 1, 2020, procedure code 90694 is covered for its FDA-approved indications. Pharmacy doesn’t require preauthorization of this drug. |
Established Experimental C1849, Q4227, Q4228, Q4229, Q4230, Q4231, q4232, Q4233, Q4234, Q4235, Q4236, Q4237, Q4239, Q4240, Q4241, Q4242, Q4244, Q4245, Q4246, Q4247, Q4248 |
Basic benefit and medical policy Amniotic membrane and amniotic fluid The listed new procedures have been added to the Amniotic Membrane and Amniotic Fluid policy. Procedure code Q4187 has been added as a payable service. The policy is effective Jan. 1, 2021. Inclusions: Treatment of nonhealing** diabetic lower extremity/venous stasis ulcers using the following human amniotic membrane products:
**Nonhealing is defined as less than a 20% decrease in wound area with standard wound care for at least two weeks. Human amniotic membrane grafts with or without suture (Prokera, AmbioDisk™) for the treatment of any of the following ophthalmic indications:
Human amniotic membrane grafts with suture or glue for the treatment of any of the following ophthalmic indications:
aConservative treatment is defined as use of topical lubricants or topical antibiotics or therapeutic contact lens or patching. b
Exclusions: All other human amniotic membrane products and indications not outlined under inclusions, including but not limited to:
|
| UPDATES TO PAYABLE PROCEDURES | |
J3490 J3590 |
Basic benefit and medical policy Xaracoll (bupivacaine hydrochloride) Xaracoll (bupivacaine hydrochloride) implant is payable when billed for the FDA‑approved indications, effective Sept. 1, 2020. Xaracoll (bupivacaine hydrochloride) implant should be reported with procedure code J3490 or J3590 and the appropriate national drug code until a permanent code is established. URMBT groups are excluded from coverage of this drug. Xaracoll (bupivacaine hydrochloride) implant contains an amide local anesthetic and is indicated in adults for placement into the surgical site to produce postsurgical analgesia for up to 24 hours following open inguinal hernia repair. Dosage and administration: Xaracoll is intended for single‑dose administration. The recommended dose is 300 mg bupivacaine HCl (three Xaracoll implants, each containing 100 mg bupivacaine HCl). Dosage forms and strengths:
|
J9999 |
Basic benefit and medical policy Blenrep (belantamab mafodotin‑blmf) Blenrep (belantamab mafodotin‑blmf) is payable when billed for the FDA‑approved indications, effective Aug. 5, 2020. Blenrep (belantamab mafodotin‑blmf) should be reported with procedure code J9999 and the appropriate national drug code until a permanent code is established. URMBT groups are excluded from coverage of this drug. Blenrep (belantamab mafodotin‑blmf) is a B‑cell maturation antigen-directed antibody and microtubule inhibitor conjugate indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies including an anti‑CD38 monoclonal antibody, a proteasome inhibitor and an immunomodulatory agent. Dosage and administration: The recommended dosage is 2.5 mg/kg as an intravenous infusion over approximately 30 minutes once every three weeks. Dosage forms and strengths: For injection: 100 mg as a lyophilized powder in a single‑dose vial for reconstitution and further dilution. |
| POLICY CLARIFICATIONS | |
0652 |
Basic benefit and medical policy Minimum number of hours for continuous hospice home care service lowered to 4 hours, effective March 1 Blue Cross Blue Shield of Michigan has lowered the minimum number of hours for continuous hospice home care service to four hours (instead of eight) and will no longer pay the daily per-diem rate. Instead, continuous home care is only paid at the hourly rate. This change is effective March 1, 2021. |
32701, 77300, 77520, 77522, 77523, 77525 |
Basic benefit and medical policy Charged‑particle (proton or helium ion) radiotherapy for neoplastic conditions Charged‑particle irradiation with proton or helium ion beams may be considered established for specific patient populations. It’s a useful therapeutic option when indicated. If safe and effective, charged‑particle irradiation with proton or helium ion beams may be an option for individual consideration in the treatment of cancer based on the analysis of dosimetric data, including comparative models if necessary. Other applications of charged‑particle irradiation with proton beams are considered experimental. Note: There is insufficient evidence to show that PBRT provides an incremental benefit in the treatment of localized prostate cancer when compared to lower cost alternative procedures. Inclusionary criteria have been updated, effective March 1, 2021. Payment policy: Use of proton beam therapy may require prior authorization to verify that Blue Cross Blue Shield of Michigan and Blue Care Network criteria are met and, where appropriate, to explore the appropriateness of using alternative therapeutic modalities such as IMRT and three‑dimensional conformal radiation therapy. Inclusions: Charged‑particle irradiation with proton or helium ion beams is established for the following indications:
Exclusions:
|
61796, 61797, 61798, 61799, 61800, 63620, 63621, 77371, 77372, 77373, G0339, G0340 |
Basic benefit and medical policy Stereotactic radiosurgery and stereotactic body radiotherapy The medical policy statement and criteria have been updated to reference AIM Specialty Health® radiology criteria, effective Nov. 1, 2020. AIM is an independent company that manages authorizations of select services for Blue Cross Blue Shield of Michigan. To reflect these updates, non-payable diagnoses on the procedures listed will be removed. Medical policy statement: The safety and effectiveness of stereotactic radiosurgery and stereotactic body radiotherapy using gamma‑ray or linear‑accelerator units are established and are considered useful therapeutic options when indicated. Reference AIM criteria for clinical preference. Inclusions: Stereotactic radiosurgery (intracranial) using a gamma‑ray or linear‑accelerator unit, or LINAC, is considered established for the following indications:
Stereotactic body radiotherapy (extracranial) is considered established for the following indications:
Stereotactic radiosurgery or stereotactic body radiotherapy using fractionation is considered established when used for indications listed above. Note:
**SBRT using proton beam therapy is acceptable for low/intermediate prostate cancer. Exclusions: Stereotactic radiosurgery and body radiotherapy are considered experimental for all other diagnoses not specified above. |
76999,** 0398T, C9734 **May be used to report unclassified service Experimental: 0071T, 0072T |
Basic benefit and medical policy Magnetic resonance‑guided focused ultrasound The safety and effectiveness of magnetic resonance-guided high‑intensity ultrasound ablation have been established. It may be a considered a useful therapeutic option in specified situations. Inclusions:
Exclusions: All other situations, including but not limited to:
|
81162, 81163, 81164, 81165, 81166, 81167, 81212, 81215, 81216, 81217 Not covered: **May be used to report unclassified service |
Basic benefit and medical policy BRCA1/BRCA2 testing for hereditary breast or ovarian cancer The safety and effectiveness of simultaneous testing for inherited BRCA1 and BRCA2 variants have been established. It may be considered a useful diagnostic option when indicated for individuals at high‑risk of breast or ovarian cancer. Testing for genomic rearrangements of the BRCA1 and BRCA2 genes (e.g., BART testing) may be considered established in patients who meet criteria for BRCA1 and BRCA2 testing and whose testing for point variants is negative. Use of multi‑gene panels, including but not limited to BreastNext, OvaNext, BRCAplus, iGene Cancer Panel and BROCA tests, is experimental. There is insufficient data on the analytical and clinical validity as well as clinical utility of these tests on patient management and outcomes. Inclusionary guidelines have been updated, effective March 1, 2021. It’s highly recommended that genetic testing should be performed in a setting that has suitably trained health care providers who can give appropriate pre‑ and post‑test counseling and that has access to a Clinical Laboratory Improvement Amendments, or CLIA, licensed laboratory that offers comprehensive variant analysis. Note:
Inclusions: Patients with cancer or with a personal history of cancer (affected patients): Genetic testing for BRCA1 and BRCA2 variants in cancer‑affected individuals may be considered appropriate under any of the following circumstances:
Patients without cancer or without a history of cancer Genetic testing for BRCA1/BRCA2 variants of cancer‑unaffected individuals may be appropriate under the following circumstances:
Exclusions:
Note: Definitions and additional background information can be found in the medical policy. |
81235, 81275, 81404, 81405, 81406, 81445, 81479 |
Basic benefit and medical policy Molecular analysis for targeted therapy or immunotherapy of non‑small cell lung cancer New genes and indications have been approved, effective March 1, 2021 EGFR testing
ALK testing
BRAF V600E testing
ROS1 testing
KRAS testing
HER2 testing
NTRK gene fusion testing
RET rearrangement testing
MET exon 14 skipping alteration
PD‑L1 testing
Tumor mutational burden testing May be established for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden‑high (TMB‑H) [≥10 mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA‑approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options (example. Keytruda). |
81327, 81382, 81479,** 82397, 82784, 83520, 84999,** 86021, 86140, 86255, 87045, 87046, 87075, 87102, 87177, 87209, 87328, 87329, 87336, 87798, 88346, 88350 **May be used to report unclassified service |
Basic benefit and medical policy Miscellaneous genetic and molecular diagnostic tests The list of excluded tests has been modified, effective March 1, 2021. Diagnostic, prognostic and therapeutic genetic testing of (1) an affected (symptomatic) individual’s germline to benefit the individual (excluding reproductive testing) or (2) of an asymptomatic individual to determine future risk of disease is considered experimental for the following:
|
81415, 81416, 81417 Experimental: 81277, 81425, 81426, 81427, 0036U |
Basic benefit and medical policy Whole exome/whole genome sequencing for diagnosis of genetic disorders Updates made to the medical policy statement are effective March 1, 2021. Whole exome sequencing, or WES, may be considered established for the evaluation of unexplained congenital or neurodevelopmental disorders in children when all the following criteria are met:
Rapid whole exome sequencing or rapid whole genome sequencing, with trio testing when possible, may be considered established for the evaluation of critically ill infants and children in neonatal or pediatric intensive care with a suspected genetic disorder of unknown etiology when at least one of the following criteria is met:
Whole genome sequencing, or WGS, is considered experimental for the diagnosis of genetic disorders. WES and WGS are considered experimental for screening for genetic disorders. Exclusions: Rapid whole exome sequencing or rapid whole genome sequencing, with trio testing when possible, isn’t established for the evaluation of critically ill infants and children in neonatal or pediatric intensive care with a suspected genetic disorder of unknown etiology in cases where:
|
83006, 0055U |
Basic benefit and medical policy Molecular testing for CHF and heart transplant The use of the Presage ST2 Assay to (1) evaluate the prognosis of patients diagnosed with chronic heart failure, (2) guide management (e.g., pharmacological, device‑based, exercise) of patients diagnosed with chronic heart failure or (3) in the post cardiac transplantation period, including but not limited to predicting prognosis and predicting acute cellular rejection is considered experimental. The use of myTAIHEART assay in the post cardiac transplantation period to predict (1) prognosis or (2) acute cellular rejection is considered experimental. This policy is effective March 1, 2021. |
95782, 95783, 95800, 95805, 95806, 95807, 95808, 95810, 95811, E0486, G0398, G0399 Not covered: 95801, A7047, E0485, E1399, G0400 |
Basic benefit and medical policy Diagnosis and medical management of sleep disorders The inclusionary and exclusionary criteria regarding oral appliances for obstructive sleep apnea have been updated, effective March 1, 2021. Medical policy statement: Diagnosis Polysomnography, or PSG, is an attended (supervised) sleep study (sleep apnea test) performed in a hospital or freestanding sleep laboratory. The safety and effectiveness of PSG, including a split-night PSG, have been established. It may be considered a useful diagnostic option when indicated. The safety and effectiveness of an unattended sleep study/sleep apnea test with a minimum of three recording channels (using, at a minimum, the following sensors: nasal pressure with chest and abdominal respiratory inductance plethysmography and oximetry; or using Peripheral Arterial Tone, known as PAT, with oximetry and actigraphy) in a home setting (home sleep study/home sleep apnea test) have been established. It may be considered a useful diagnostic option when indicated. The safety and effectiveness of multiple sleep latency testing, or MSLT, have been established. It may be a useful tool in diagnosing narcolepsy. Noninvasive pulse oximetry as a sole test (as an alternative to polysomnography or as a cardiorespiratory study for diagnosing sleep related breathing disorders) is considered experimental. Its effectiveness hasn’t been established. Medical management The safety and effectiveness of oral appliances to reduce upper airway collapsibility in the treatment of OSA have been established. An oral appliance may be considered a useful therapeutic option when indicated. Palate and mandible expansion devices are considered experimental for the treatment of OSA. There is insufficient evidence in the current medical literature to support their efficacy and use in clinical practice. Nasal Expiratory Positive Airway Pressure (nasal EPAP) for the treatment of OSA is considered experimental. There is insufficient evidence in the current medical literature to support its efficacy and use in clinical practice. Oral pressure therapy for the treatment of OSA is considered experimental. There is insufficient medical literature found to support its efficacy. Positional therapy devices, such as the NightBalance Lunoa SPT system, are considered experimental. They haven’t been proven to be more effective than standard care. Inclusions: Note: Due to the length of the complete policy guidelines, only the updates are included here. Below are the changes that were made to the medical management section regarding oral appliances for obstructive sleep apnea: Oral appliances for OSA (e.g., tongue‑retaining devices or mandibular orthopedic positioning devices) may be considered established in adult patients with clinically significant OSA. (Verify coverage of intraoral appliances under the DME benefit.) Definition of an oral appliance for OSA
An appropriate oral appliance will allow for optimal protrusion of the mandible (e.g., less than 5 mm) to produce the desired relative opening of the airway, without contributing to an increased risk of temporal mandibular joint dysfunction. Inclusions (includes all the following):
Replacement of an oral appliance may be considered at the end of the five-year reasonable useful lifetime or prior if there’s a change in the patient’s condition. Exclusions:
Palate and mandible expansion devices are considered experimental. Nasal expiratory positive airway pressure is considered experimental. Oral pressure therapy is considered experimental. Positional therapy devices, such as the NightBalance Lunoa SPT system, are considered experimental. They haven’t been proven to be more effective than standard care. Reference the medical policy for complete coverage guidelines on other services related to the diagnosis and management of obstructive sleep apnea. |
J0490 |
Basic benefit and medical policy Benlysta (belimumab) Effective Dec. 16, 2020, Benlysta (belimumab) is covered for the following updated FDA‑approved indications: Adult patients with active lupus nephritis who are receiving standard therapy. |
J0588 |
Basic benefit and medical policy Xeomin (incobotulinumtoxinA) Effective Dec. 18, 2020, Xeomin (incobotulinumtoxinA) is payable for the following updated FDA‑approved indications:
Dosage and administration:
|
J3490 J3590 |
Basic benefit and medical policy Riabni (rituximab-arrx) Effective Dec. 17, 2020, Riabni (rituximab‑arrx) is covered for the following FDA-approved indications: Riabni (rituximab‑arrx) is a CD20‑directed cytolytic antibody indicated for the treatment of:
Dosage and administration:
Dosage forms and strengths:
This drug isn’t a benefit for URMBT. |
J9203 |
Basic benefit and medical policy Mylotarg (gemtuzumab ozogamicin) Mylotarg (gemtuzumab ozogamicin) is approved for the following updated FDA indication: Treatment of newly diagnosed CD33‑positive acute myeloid leukemia in adults and pediatric patients 1 month and older |
J9312 |
Basic benefit and medical policy J9312 payable for treatment of multiple sclerosis Procedure code J9312 is payable for the treatment of multiple sclerosis (ICD‑10 G35). The ICD‑10 diagnosis code is payable in addition to those diagnosis codes already payable. |
| EXPERIMENTAL PROCEDURES | |
22899,** C9752, C9753 **Used to report unclassified procedure |
Basic benefit and medical policy RF ablation of basivertebral nerve for low back pain (e.g., Intracept®) Radiofrequency ablation of the basivertebral nerve is considered experimental. There is insufficient evidence to determine if radiofrequency ablation of the basivertebral nerve improves health outcomes. This policy is effective March 1, 2021. |
![]()
Additional drugs covered under Medicare Part B now available at retail pharmacies
Beginning in April, pharmacies can begin billing Medicare Plus Blue℠ plans directly for certain drugs approved for coverage under the Medicare Part B benefit. Previously, many retail pharmacies were unable to bill Part B medications directly and often charged members in full for these drugs. This required members to complete and submit a reimbursement form to Blue Cross Blue Shield of Michigan.
Any existing cost share for these drugs still applies, according to the member’s plan.
The table below lists the medication types and how they will be processed.
| Drug type | What’s new | Medicare Advantage Plan(s) included |
|---|---|---|
| Nebulizer solutions | Will automatically process under Part B if member lives at home and under Part D if member resides in a long-term care or skilled nursing facility |
Medicare Plus Blue℠ plans with or without prescription coverage if Blue Cross ID card used** |
| Select oral cancer medications | Will process under Part B; no prior authorization required |
Medicare Plus Blue℠ plans with or without prescription coverage if Blue Cross ID card used |
| Oral antiemetics and immunosuppressants | Will process under the appropriate benefit once a coverage review of Part B versus Part D is complete |
Medicare Plus Blue℠ plans with prescription coverage*** |
**For members without a Medicare Plus Blue prescription drug plan who reside in a long‑term care or skilled nursing facility, bill using their Part D plan ID card.
***For members without a Medicare Plus Blue prescription drug plan, if the oral antiemetic or immunosuppressant is approved for Part B coverage, bill using the CMS 1500 form.
View the lists of Medicare Part B drugs available at point of service depending on the member’s plan as follows:
Medicare Plus Blue only
Medicare Plus Blue with prescription drug coverage
Exclusions
Prescription Blue℠ PDP members are excluded from this program.
Additional medical benefit drugs to require prior authorization for some commercial members
What you need to know
For dates of service on or after May 24, 2021, you’ll need to submit prior authorization requests to AIM Specialty Health® for Herceptin Hylecta™ (trastuzumab and hyaluronidase‑oysk), Herzuma® (trastuzumab‑pkrb), Ogivri™ (trastuzumab‑dkst) and Trazimera™ (trastuzumab‑qyyp) for some commercial members.
Blue Cross Blue Shield of Michigan will add prior authorization requirements for additional drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust non‑Medicare members.
For dates of service on or after May 24, 2021, submit prior authorization requests to AIM Specialty Health® for these drugs:
- Herceptin Hylecta™ (trastuzumab and hyaluronidase‑oysk), HCPCS code J9356
- Herzuma® (trastuzumab‑pkrb), HCPCS code Q5113
- Ogivri™ (trastuzumab‑dkst), HCPCS code Q5114
- Trazimera™ (trastuzumab‑qyyp), HCPCS code Q5116
How to submit requests
Submit requests through the AIM ProviderPortal** or by calling the AIM Contact Center at 1‑844‑377‑1278.
For information about registering for and accessing the AIM ProviderPortal, see the Frequently Asked Questions page** on the AIM website.
More about the authorization requirements
Authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
For more information on requirements related to drugs covered under the medical benefit for these members, refer to the Medical oncology prior authorization list for UAW Retiree Medical Benefit Trust PPO non‑Medicare members. We’ll update this list to reflect these changes prior to the effective date.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Blue Cross and BCN ending COVID‑19 anti‑stockpiling quantity limits
What you need to know
- Temporary quantity limits on select medications are ending April 1.
- We’re also making changes to quantity limits for albuterol inhalers.
- We’re reminding you of how to submit quantity limit waiver requests.
On April 1, 2021, Blue Cross Blue Shield of Michigan and Blue Care Network will end temporary anti‑stockpiling quantity limits on select medications used to treat COVID‑19 symptoms.
We adopted temporary quantity limits in April 2020 on select medications to prevent unnecessary stockpiling during the COVID‑19 public health crisis and to ensure access for members who need these medications to treat their chronic conditions. This was announced in an April 30, 2020, provider alert.
Quantity limits will return to what they were before the temporary COVID‑19 policy, with the exception of albuterol inhalers. We previously covered four albuterol inhalers per 30 days. Effective April 1, we’ll cover two inhalers per 30 days. This change isn’t related to the COVID‑19 public health crisis. Consult the Temporary quantity limits on some prescription drugs during the COVID‑19 pandemic document for details, including instructions on how to request a quantity limit waiver if you have a patient who needs to exceed the albuterol inhaler limit. Physicians requesting more than two inhalers per month for a patient can follow the usual process for requesting an exception to the quality limits.
For information on changes related to the COVID‑19 pandemic, see the Temporary changes due to the COVID‑19 pandemic document. It’s on our coronavirus webpage, which is available through Provider Secured Services and on our public website at bcbsm.com/coronavirus (click on the Health Care Providers tab).
How to submit quantity limit waiver requests
Physicians can submit quantity limit waiver requests through our standard prior authorization processes.
To request authorization in writing, fax a Medication Request Form — Quantity Limit Request to the Blue Cross Pharmacy Clinical Help Desk at 1‑866‑601‑4425. To find the form:
- Log in to web‑DENIS.
- Click on BCBSM Provider Publications and Resources.
- Click on Commercial Pharmacy Prior Authorization and Step Therapy forms.
- Click on Quantity Limit Request.
If you have any questions, call the Pharmacy Clinical Help Desk at 1‑800‑437‑3803.
Shingles vaccine recommendation updated
National health care reform policy has been updated to include the following recommendation for the shingles vaccine, procedure code *90750:
- Payable for groups subject to national health care reform policy without cost share
- Adults 50 years and older to receive two doses of the shingles vaccine two to six months apart
Breyanzi requires prior authorization for Medicare Advantage members
For Medicare Plus Blue℠ and BCN Advantage℠ members, we require authorization for the CAR‑T medication Breyanzi® (lisocabtagene maraleucel), HCPCS code J9999, when administered at on‑campus or off‑campus outpatient hospitals (site of care 19 and 22).
Here’s how to bill:
- Electronically through an 837P transaction or on a professional CMS‑1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
Reminder:
Submit authorization requests for Breyanzi through NovoLogix. It offers real‑time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to enter authorization requests through NovoLogix.
If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
Reminder about ClaimsXten edits for anesthesia services
In the May 2018 edition of The Record, we published an article about ClaimsXten edits that are in place in our systems. We wanted to remind you about those ClaimsXten edits, particularly the edits for anesthesia services.
Anesthesia services reported with non‑anesthesia codes that aren’t eligible to be reported for anesthesia providers will be denied and will need to be resubmitted with the correct anesthesia code.
The edits are designed to promote correct coding and simplify our claims payment systems.
Virtual provider symposiums to focus on patient experience, HEDIS, documentation and coding
Action item
Register for one or more of the upcoming provider symposiums, using the links included in this article.
We’ve scheduled this year’s provider symposiums virtually throughout May and June for physicians, office staff and coders. The dates are listed below. You may register by clicking on the registration links, and you may register for more than one topic.
These sessions are for physicians and office staff responsible for closing gaps in care related to quality measures and creating a positive patient experience:
| Topic | Date and time | Registration link |
|---|---|---|
| HEDIS® measures (details and exclusions), Consumer Assessment of Healthcare Providers and Systems Survey and Health Outcomes Survey | Tuesday, May 4 |
Register here |
| HEDIS® measures (details and exclusions), Consumer Assessment of Healthcare Providers and Systems Survey and Health Outcomes Survey | Wednesday, May 12 |
Register here |
| HEDIS® measures (details and exclusions), Consumer Assessment of Healthcare Providers and Systems Surveyand Health Outcomes Survey | Thursday, May 20 |
Register here |
| HEDIS® measures (details and exclusions), Consumer Assessment of Healthcare Providers and Systems Survey and Health Outcomes Survey | Tuesday, June 8 |
Register here |
| HEDIS® measures (details and exclusions), Consumer Assessment of Healthcare Providers and Systems Surveyand Health Outcomes Survey | Wednesday, June 16 |
Register here |
| HEDIS® measures (details and exclusions), Consumer Assessment of Healthcare Providers and Systems Surveyand Health Outcomes Survey | Thursday, June 24 |
Register here |
| Patient experience top 5: Let’s take a poll! | Tuesday, May 4 |
Register here |
| Patient experience top 5: Let’s take a poll! | Wednesday, May 5 |
Register here |
| Patient experience top 5: Let’s take a poll! | Wednesday, May 12 |
Register here |
| Patient experience top 5: Let’s take a poll! | Thursday, May 20 |
Register here |
| Patient experience top 5: Let’s take a poll! | Tuesday, June 8 |
Register here |
| Patient experience top 5: Let’s take a poll! | Wednesday, June 16 |
Register here |
| Updates on CPT, ICD‑10‑CM, evaluation and management codes and coding for social determinants of health, or SDOH | Thursday, May 6 |
Register here |
| Updates on CPT, ICD‑10‑CM, evaluation and management codes and coding for social determinants of health, or SDOH | Tuesday, May 11 |
Register here |
| Updates on CPT, ICD‑10‑CM, evaluation and management codes and coding for social determinants of health, or SDOH | Wednesday, May 19 |
Register here |
| Updates on CPT, ICD‑10‑CM, evaluation and management codes and coding for social determinants of health, or SDOH | Thursday, June 10 |
Register here |
| Updates on CPT, ICD‑10‑CM, evaluation and management codes and coding for social determinants of health, or SDOH | Tuesday, June 15 |
Register here |
| Updates on CPT, ICD‑10‑CM, evaluation and management codes and coding for social determinants of health, or SDOH | Wednesday, June 23 |
Register here |
Physicians, physician assistants, nurse practitioners, nurses and coders can receive continuing education credits for attending the sessions.
HEDIS® is a registered trademark of the National Committee for Quality Assurance.
Lunch and learn webinars for physicians and coders focus on risk adjustment, coding
Action item
Sign up now for our live, monthly, lunchtime webinars.
Starting this month, we’re offering webinars that will provide updated information on documentation and coding of common challenging diagnoses. These live, lunchtime educational sessions will also include an opportunity to ask any questions you may have.
The April through September webinars are led by physicians. The last three sessions of the year focus on coding guideline updates and are led by coders.
Here’s our current schedule and the tentative topics. All sessions start at 12:15 p.m. Eastern time and generally run for 15 to 30 minutes. Click on a Register here link below to sign up for a session.
| Session date | Topic | Sign‑up link |
|---|---|---|
| Tuesday, April 20 | Acute conditions reported in the outpatient setting | Register here |
| Wednesday, May 19 | Morbid (severe) obesity | Register here |
| Thursday, June 17 | Major depression | Register here |
| Tuesday, July 20 | Diabetes with complications | Register here |
| Wednesday, Aug. 18 | Renal disease | Register here |
| Thursday, Sept. 23 | Malignant neoplasm | Register here |
| Tuesday, Oct. 12 | Updates for ICD‑10‑CM | Register here |
| Wednesday, Nov. 17 | Coding scenarios for primary care and specialty | Register here |
| Thursday, Dec. 9 | E/M coding tips | Register here |
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding registration, email Patricia Scarlett at pscarlett@bcbsm.com.
Latest on‑demand training available now
Action item
Check out what’s new on our provider training websites, including an e‑learning overview of the Blue High Performance Network, a webinar on autism services and a guide for athletic trainers.
Provider Experience offers training resources for health care providers and staff. We’ve posted recordings of webinars delivered so far in 2021. Video and e‑learning modules are also available on specific topics.
The on‑demand courses are designed to help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network.
Some of the newest resources include:
- Blue High Performance Network e‑Learning: This video gives you an overview of the new Blue High Performance Network℠ to help providers care for patients. Note that this is only posted on the BCBSM Provider Training site.
- Autism services overview: This recorded webinar reviews current processes related to delivering services to members with autism.
- Provider training and resource guide for athletic trainers: This is a new document that shares links to training modules and resources for newly contracted athletic trainers.
Recordings of previous webinars are available on web‑DENIS through BCBSM Provider Publications and Resources or BCN Provider Publications and Resources as follows:
Blue Cross Provider Publications and Resources
- Log in to Provider Secured Services.
- Click on BCBSM Provider Publications and Resources.
- Click on Newsletters & Resources.
- In the Popular Links section, click on Provider Training.
- In the Featured Links section of the main page, check out the latest 2021 provider training webinars.
- To find video and e‑learning modules, click on E‑Learning (Online training, presentations and videos). It’s at the top of the page in the Quick Access section.
You can also get more information about online training, presentations and videos by clicking on the Quick Links E‑Learning (Online training, presentations and videos) icon.
BCN Provider Publications and Resources
- Log in to Provider Secured Services.
- Go to BCN Provider Publications and Resources.
- Under Other Resources, click on Learning Opportunities.
- Scroll down to find the most recent webinars under 2021 Provider Training Webinars.
As additional training webinars become available, we’ll provide notices through
web‑DENIS, The Record and BCN Provider News.
New provider training website coming later this year
To improve the training experience for health care providers and staff, Provider Experience is launching a new training website this year. The new site will help you easily find training resources, such as recorded webinars, videos, e‑learning modules and supporting documents.
In future Record articles, we’ll share details on the launch date, requesting access and how to navigate the site and track progress.
Blue Cross changing practitioner fees July 1
Blue Cross Blue Shield of Michigan will change practitioner fees for services with dates of service on or after July 1, 2021. This change applies to services provided to our Traditional, TRUST and Blue Preferred Plus℠ members, regardless of customer group.
Blue Cross will use the 2021 Medicare resource-based relative value scale for most relative value unit‑priced procedures for dates of service on and after July 1. Most fees are currently priced using the 2020 values.
In addition, the conversion factor used to calculate approved amounts in Blue Cross’ anesthesia fee structure will increase to $65.50.
The Blue Cross overall fee increase of 2.25% includes base fee adjustments and value‑based reimbursement. Due to significant changes in relative value unit, or RVU, valuations, it’s best to review the fee schedule to view the effect on an individual code or group of codes.
Fee schedules effective July 1 will be available on web‑DENIS on April 1. To find fee schedule information, go to the homepage of web‑DENIS and follow these steps:
- Click on BCBSM Provider Publications and Resources.
- Click on Entire Fee Schedules and Fee Changes, which will bring up the End User Agreement.
- Click on Accept.
Only claims submitted with dates of service on or after July 1 will be reimbursed at the new rates.
PGIP Risk‑Bearing OSC program ends June 30
Effective June 30, 2021, the Physician Group Incentive Program Risk‑Bearing Organized Systems of Care program will be ending. Blue Cross Blue Shield of Michigan first announced the 2% allocation in the January 2019 issue of The Record.
The program focused on rewarding PGIP OSCs for managing the benefit cost trend of their attributed Blue Cross PPO patient population, and prepared providers to take on risk associated with the Blueprint for Affordability program.
Changes coming to PGIP allocation to support chronic disease management efforts
To better support Collaborative Quality Initiatives that address chronic disease management and common surgical procedures, the Physician Group Incentive Program allocation will change to 6%, effective July 1, 2021. This change is for all provider types and procedure codes that are subject to the allocation and is intended to support our health care providers and members.
All funds allocated are distributed to eligible organizations that participate in PGIP to support physician practice and system transformation. No money is retained by Blue Cross Blue Shield of Michigan for administrative costs.
Visit bcbsm.com/provider/valuepartnerships/pgip for more information about PGIP.
Note: Claims for Federal Employee Program® members are excluded from the PGIP allocation.
HEDIS and Star tip sheets updated for 2021
We’ve updated our HEDIS® tip sheets** for 2021 and posted them on the Clinical Quality Corner page of web‑DENIS, along with a series of Star Measure Tips. The tip sheets were developed to assist health care providers and their staff in their efforts to improve overall health care quality and prevent or control diseases and chronic conditions.
The new 2021 tip sheets that have been posted are up to date as of this publication. As updated versions are produced, we’ll post new ones and announce them in The Record. For example, after the National Committee for Quality Assurance publishes final updates to the 2021 HEDIS specifications, we may need to update the tip sheets again.
The Star Measure Tips highlight select measures in the Medicare Star Ratings program. Most of the measures featured in the Star Measure Tips are also HEDIS measures. HEDIS is one of the most widely used performance improvement tools in the U.S.
Accessing the tip sheets
These HEDIS Measure Tip Sheets and the Star Measure Tips are housed on the Clinical Quality Corner page of web‑DENIS. You can get there by following these steps:
- From the homepage of web‑DENIS, click on BCBSM Provider Publications and Resources in the left column. (You can also access them from the BCN Provider Publications and Resources section of web‑DENIS.)
- Click on Newsletters & Resources.
- Click on Clinical Quality Corner on the left-hand side of the page under Other Resources.
**HEDIS®, which stands for Healthcare Effectiveness Data and Information Set, is a registered trademark of the National Committee for Quality Assurance, or NCQA.
Advanced illness, frailty exclusions allowed for select HEDIS measures
Action item
Check out the HEDIS® Advanced Illness and Frailty Exclusions Guide that we link to in this article.
The National Committee for Quality Assurance allows patients to be excluded from select HEDIS quality measures due to advanced illness and frailty. The NCQA acknowledges** that measured services most likely would not benefit patients who are in declining health.
Health care providers may submit claims with advanced illness and frailty codes to exclude patients from select measures. Using these codes also reduces medical record requests for HEDIS data collection purposes.
Read the HEDIS® Advanced Illness and Frailty Exclusions Guide for a description of the advanced illness and frailty exclusion criteria and a list with some of the appropriate HEDIS‑approved billing codes.
HEDIS®, which stands for Healthcare Effectiveness Data Information Set, is a registered trademark of the National Committee for Quality Assurance.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Federal Employee Program offers tools to help FEP members manage their heart health
This is part of an ongoing series of articles focusing on the tools and resources available to help FEP members manage their health.
Did you know … In the U.S. about 655,000 people die from heart disease each year, according to the Centers for Disease Control and Prevention. That’s 1 in every 4 deaths in the country. With support from health care providers, patients can protect their heart health by taking an active role in managing chronic conditions that can lead to heart disease such as high blood pressure, high cholesterol and diabetes.
The Federal Employee Program® Service Benefit Plan offers members tools and support to complement their health care providers’ treatment plans so they can take an active role in managing their heart health. Resources include:
- The Blue Health Assessment is a health and lifestyle questionnaire that helps members set goals, receive supportive advice and track their progress.
- The MyBlue® account, www.fepblue.org/myblue, provides members with online health coaching, access to a personal health record for tracking medications, test results, medical appointments and educational tools on a wide range of health‑related topics.
- Flyers are available for quick, easy reference on controlling high blood pressure and managing diabetes. Information on cholesterol** levels can be accessed from the American Heart Association website.
Members can find more online tools at www.fepblue.org.
Programs available to FEP members include:
- Hypertension Management Program (all FEP members)
- Online: www.fepblue.org/hypertension
- Phone: 1‑888‑258‑3432
- Diabetes Management Program (Standard Option and Basic Option Plan FEP members only)
- Online: www.fepblue.org/diabetes
- Phone: 1‑800‑593‑8490
- Diabetes Incentive Program (Standard Option and Basic Option Plan FEP members only)
- Online: www.fepblue.org/diabetes
- Phone: 1‑800‑593‑8490
- Coordinated Care Program
- Phone: 1‑800‑775‑2583
For more information about FEP services and benefits, members and providers can call Customer Service at 1‑800‑482‑3600.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Clarification: Blue Cross has selected Northwood as its DME/POS benefit manager
What you need to know
- Participating in the Northwood network may result in more limited provider options for some members.
- The move to the Northwood network also applies to members who receive supplies or devices directly from the manufacturer.
Effective Jan. 1, 2021, Blue Cross Blue Shield of Michigan selected Northwood Inc., as its durable medical equipment, prosthetics, orthotics and medical supplies benefit manager for Blue Cross Blue Shield of Michigan fully insured commercial and individual members who reside in Michigan.
Affected members were notified about the change by letter or email in October 2020. The notification included information about how to locate a Northwood provider. Members are encouraged to use network providers through the Northwood network to help keep costs as low as possible.
Keep in mind that participating in the Northwood network may result in more limited provider options for some members. They may need to travel farther to obtain a specific brand or device or they may need to find a replacement brand or device not available with their local provider.
We wanted to clarify that this change also applies to members who receive supplies or devices directly from the manufacturer (for example, Medtronic or Dexcom). Currently, there is no process to provide exceptions to the network guidelines. We advise members to always check their benefits when considering an out‑of‑network option.
For more information about the Northwood program and how it works, refer to the Northwood DMEPOS Management Program FAQ and the Northwood DMEPOS Management Program Procedure Code Requiring Prior Authorization documents located on bcbsm.com/providers.
If you have questions about the Northwood DME/POS management program, contact Northwood Provider Relations at 1‑800‑447‑9599 between 8:30 a.m. and 5 p.m. Eastern time Monday through Friday.
Blue Elect Plus members don’t need referrals
What you need to know
We’re including this information in The Record so that specialists who participate with Blue Cross Blue Shield of Michigan know that members who have this BCN plan don’t need a referral to see them.
Health care providers should be aware that members enrolled in Blue Care Network’s Blue Elect Plus℠ point‑of‑service plan don’t need referrals to see a specialist.
When a patient calls a specialist for an appointment, their office staff needs to verify whether the member has BCN HMO or the Blue Elect Plus POS coverage. Keep in mind that BCN members can have HMO or POS benefits.
Blue Elect Plus is a point‑of‑service plan that doesn’t require referrals to see a specialist, either in or out of network. The member ID card prefix is the same prefix that’s on the member ID card for HMO coverage. But the plastic member ID card specifically indicates “POS” coverage.
In addition, wording on the back of the member ID card notes that referrals aren’t required for Blue Elect Plus. By contrast, the virtual member ID card doesn’t indicate that the member is in a point-of-service plan. That’s why it’s always important to check web‑DENIS for eligibility and benefits.
For Blue Elect Plus, some services, including most preventive care, are only covered when received from a network provider. Providers should also be aware that some services require prior authorization.
Billing policy and guidelines for intensity modulated radiation therapy
When billing for intensity modulated radiation therapy, or IMRT, the following guidelines should be followed, in accordance with a new Blue Cross Blue Shield of Michigan policy. This policy has been adopted to align billing requirements with industry and Centers for Medicare & Medicaid Services standards.
When an IMRT simulation is performed on the same tumor within 14 days before an IMRT plan, reimbursement of the simulation will be included in the reimbursement whether the simulation is reported on the same or different date of service. In addition, the IMRT policy addresses certain radiation therapy services that may be performed 14 days before, on, or as part of the development of the IMRT plan.
In accordance with the American Medical Association and CMS’ National Correct Coding Initiative Policy Manual, Blue Cross considers CPT codes *77014, *77280, *77285, *77290, *77295, *77306 through *77321, *77331 and *77370 as included in the payment for CPT code *77301 (IMRT planning) when performed in the development of the IMRT plan on the same or different dates of service for the same tumor. To report services for a different tumor on a different date of service, use the appropriate modifier to identify that it is separate, distinct and unrelated to the IMRT plan.
IMRT simulation services billed separately and not billed according to the above guidelines will be denied.
Reminder to update your Provider Authorization form when changes occur
Blue Cross Blue Shield of Michigan is dedicated to safeguarding the protected health information of its members. These safeguards include completion of a Trading Partner Agreement and Provider Authorization form as part of the electronic data interchange setup process. All EDI trading partners must complete a TPA and Provider Authorization form before they can exchange PHI with Blue Cross.
The terms of the TPA require you to notify Blue Cross of any changes in your trading partner information, so it’s important to keep your Provider Authorization form up to date. Updating the form also ensures information is routed to the proper destination.
Update the form when you switch:
- Service bureaus or clearinghouses
- Software vendors
- Billing services
- 835‑file recipients
- Submitter IDs
- Unique 835 receivers or Trading Partner IDs
Review the form when you:
- Join a new group practice
- Leave a group practice and start billing using your own NPI
- Hire a new billing service
- Start submitting claims through a clearinghouse or when you’ve changed clearinghouses
- Decide you no longer want to receive 835 remittance files
- Select a new destination for your 835
You don’t need to update the Provider Authorization form if your submitter and Trading Partner IDs don’t change.
How to change your EDI setup
To make changes to your EDI setup log in to the Trading Partner Agreement webpage.
Or follow the complete navigation steps to arrive at the TPA login page:
- Visit bcbsm.com/providers
- Scroll down to the Provider resources section and click on Quick Links.
- Click on Electronic Connectivity EDI.
- Click on How to use EDI to exchange information with us electronically.
- Scroll down to the EDI agreements section and click on Update your Provider Authorization Form.
If you have any questions about EDI enrollment, contact the EDI Help Desk at 1‑800‑542‑0945. For assistance with the TPA or the Provider Authorization form, select the TPA option.
![]()
Additional drugs covered under Medicare Part B now available at retail pharmacies
Beginning in April, pharmacies can begin billing Medicare Plus Blue℠ plans directly for certain drugs approved for coverage under the Medicare Part B benefit. Previously, many retail pharmacies were unable to bill Part B medications directly and often charged members in full for these drugs. This required members to complete and submit a reimbursement form to Blue Cross Blue Shield of Michigan.
Any existing cost share for these drugs still applies, according to the member’s plan.
The table below lists the medication types and how they will be processed.
| Drug type | What’s new | Medicare Advantage Plan(s) included |
|---|---|---|
| Nebulizer solutions | Will automatically process under Part B if member lives at home and under Part D if member resides in a long-term care or skilled nursing facility |
Medicare Plus Blue℠ plans with or without prescription coverage if Blue Cross ID card used** |
| Select oral cancer medications | Will process under Part B; no prior authorization required |
Medicare Plus Blue℠ plans with or without prescription coverage if Blue Cross ID card used |
| Oral antiemetics and immunosuppressants | Will process under the appropriate benefit once a coverage review of Part B versus Part D is complete |
Medicare Plus Blue℠ plans with prescription coverage*** |
**For members without a Medicare Plus Blue prescription drug plan who reside in a long‑term care or skilled nursing facility, bill using their Part D plan ID card.
***For members without a Medicare Plus Blue prescription drug plan, if the oral antiemetic or immunosuppressant is approved for Part B coverage, bill using the CMS 1500 form.
View the lists of Medicare Part B drugs available at point of service depending on the member’s plan as follows:
Medicare Plus Blue only
Medicare Plus Blue with prescription drug coverage
Exclusions
Prescription Blue℠ PDP members are excluded from this program.
Additional medical benefit drugs to require prior authorization for some commercial members
What you need to know
For dates of service on or after May 24, 2021, you’ll need to submit prior authorization requests to AIM Specialty Health® for Herceptin Hylecta™ (trastuzumab and hyaluronidase‑oysk), Herzuma® (trastuzumab‑pkrb), Ogivri™ (trastuzumab‑dkst) and Trazimera™ (trastuzumab‑qyyp) for some commercial members.
Blue Cross Blue Shield of Michigan will add prior authorization requirements for additional drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust non‑Medicare members.
For dates of service on or after May 24, 2021, submit prior authorization requests to AIM Specialty Health® for these drugs:
- Herceptin Hylecta™ (trastuzumab and hyaluronidase‑oysk), HCPCS code J9356
- Herzuma® (trastuzumab‑pkrb), HCPCS code Q5113
- Ogivri™ (trastuzumab‑dkst), HCPCS code Q5114
- Trazimera™ (trastuzumab‑qyyp), HCPCS code Q5116
How to submit requests
Submit requests through the AIM ProviderPortal** or by calling the AIM Contact Center at 1‑844‑377‑1278.
For information about registering for and accessing the AIM ProviderPortal, see the Frequently Asked Questions page** on the AIM website.
More about the authorization requirements
Authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
For more information on requirements related to drugs covered under the medical benefit for these members, refer to the Medical oncology prior authorization list for UAW Retiree Medical Benefit Trust PPO non‑Medicare members. We’ll update this list to reflect these changes prior to the effective date.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Blue Cross and BCN ending COVID‑19 anti‑stockpiling quantity limits
What you need to know
- Temporary quantity limits on select medications are ending April 1.
- We’re also making changes to quantity limits for albuterol inhalers.
- We’re reminding you of how to submit quantity limit waiver requests.
On April 1, 2021, Blue Cross Blue Shield of Michigan and Blue Care Network will end temporary anti‑stockpiling quantity limits on select medications used to treat COVID‑19 symptoms.
We adopted temporary quantity limits in April 2020 on select medications to prevent unnecessary stockpiling during the COVID‑19 public health crisis and to ensure access for members who need these medications to treat their chronic conditions. This was announced in an April 30, 2020, provider alert.
Quantity limits will return to what they were before the temporary COVID‑19 policy, with the exception of albuterol inhalers. We previously covered four albuterol inhalers per 30 days. Effective April 1, we’ll cover two inhalers per 30 days. This change isn’t related to the COVID‑19 public health crisis. Consult the Temporary quantity limits on some prescription drugs during the COVID‑19 pandemic document for details, including instructions on how to request a quantity limit waiver if you have a patient who needs to exceed the albuterol inhaler limit. Physicians requesting more than two inhalers per month for a patient can follow the usual process for requesting an exception to the quality limits.
For information on changes related to the COVID‑19 pandemic, see the Temporary changes due to the COVID‑19 pandemic document. It’s on our coronavirus webpage, which is available through Provider Secured Services and on our public website at bcbsm.com/coronavirus (click on the Health Care Providers tab).
How to submit quantity limit waiver requests
Physicians can submit quantity limit waiver requests through our standard prior authorization processes.
To request authorization in writing, fax a Medication Request Form — Quantity Limit Request to the Blue Cross Pharmacy Clinical Help Desk at 1‑866‑601‑4425. To find the form:
- Log in to web‑DENIS.
- Click on BCBSM Provider Publications and Resources.
- Click on Commercial Pharmacy Prior Authorization and Step Therapy forms.
- Click on Quantity Limit Request.
If you have any questions, call the Pharmacy Clinical Help Desk at 1‑800‑437‑3803.
Breyanzi requires prior authorization for Medicare Advantage members
For Medicare Plus Blue℠ and BCN Advantage℠ members, we require authorization for the CAR‑T medication Breyanzi® (lisocabtagene maraleucel), HCPCS code J9999, when administered at on‑campus or off‑campus outpatient hospitals (site of care 19 and 22).
Here’s how to bill:
- Electronically through an 837P transaction or on a professional CMS‑1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
Reminder:
Submit authorization requests for Breyanzi through NovoLogix. It offers real‑time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to enter authorization requests through NovoLogix.
If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
Lunch and learn webinars for physicians and coders focus on risk adjustment, coding
Action item
Sign up now for our live, monthly, lunchtime webinars.
Starting this month, we’re offering webinars that will provide updated information on documentation and coding of common challenging diagnoses. These live, lunchtime educational sessions will also include an opportunity to ask any questions you may have.
The April through September webinars are led by physicians. The last three sessions of the year focus on coding guideline updates and are led by coders.
Here’s our current schedule and the tentative topics. All sessions start at 12:15 p.m. Eastern time and generally run for 15 to 30 minutes. Click on a Register here link below to sign up for a session.
| Session date | Topic | Sign‑up link |
|---|---|---|
| Tuesday, April 20 | Acute conditions reported in the outpatient setting | Register here |
| Wednesday, May 19 | Morbid (severe) obesity | Register here |
| Thursday, June 17 | Major depression | Register here |
| Tuesday, July 20 | Diabetes with complications | Register here |
| Wednesday, Aug. 18 | Renal disease | Register here |
| Thursday, Sept. 23 | Malignant neoplasm | Register here |
| Tuesday, Oct. 12 | Updates for ICD‑10‑CM | Register here |
| Wednesday, Nov. 17 | Coding scenarios for primary care and specialty | Register here |
| Thursday, Dec. 9 | E/M coding tips | Register here |
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding registration, email Patricia Scarlett at pscarlett@bcbsm.com.
Latest on‑demand training available now
Action item
Check out what’s new on our provider training websites, including an e‑learning overview of the Blue High Performance Network, a webinar on autism services and a guide for athletic trainers.
Provider Experience offers training resources for health care providers and staff. We’ve posted recordings of webinars delivered so far in 2021. Video and e‑learning modules are also available on specific topics.
The on‑demand courses are designed to help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network.
Some of the newest resources include:
- Blue High Performance Network e‑Learning: This video gives you an overview of the new Blue High Performance Network℠ to help providers care for patients. Note that this is only posted on the BCBSM Provider Training site.
- Autism services overview: This recorded webinar reviews current processes related to delivering services to members with autism.
- Provider training and resource guide for athletic trainers: This is a new document that shares links to training modules and resources for newly contracted athletic trainers.
Recordings of previous webinars are available on web‑DENIS through BCBSM Provider Publications and Resources or BCN Provider Publications and Resources as follows:
Blue Cross Provider Publications and Resources
- Log in to Provider Secured Services.
- Click on BCBSM Provider Publications and Resources.
- Click on Newsletters & Resources.
- In the Popular Links section, click on Provider Training.
- In the Featured Links section of the main page, check out the latest 2021 provider training webinars.
- To find video and e‑learning modules, click on E‑Learning (Online training, presentations and videos). It’s at the top of the page in the Quick Access section.
You can also get more information about online training, presentations and videos by clicking on the Quick Links E‑Learning (Online training, presentations and videos) icon.
BCN Provider Publications and Resources
- Log in to Provider Secured Services.
- Go to BCN Provider Publications and Resources.
- Under Other Resources, click on Learning Opportunities.
- Scroll down to find the most recent webinars under 2021 Provider Training Webinars.
As additional training webinars become available, we’ll provide notices through
web‑DENIS, The Record and BCN Provider News.
New provider training website coming later this year
To improve the training experience for health care providers and staff, Provider Experience is launching a new training website this year. The new site will help you easily find training resources, such as recorded webinars, videos, e‑learning modules and supporting documents.
In future Record articles, we’ll share details on the launch date, requesting access and how to navigate the site and track progress.
There’s a new information bubble icon in Benefit Explainer
What you need to know
In February, an information bubble icon for the Medical Policy Primary Diagnosis limitation rule was added to Benefit Explainer. The new icon indicates the location of the diagnosis on the facility claim that applies to the rule.
Benefit Explainer users now see a new information bubble icon when viewing a standalone Primary Diagnosis limitation rule. The change took place on Feb. 8, 2021.
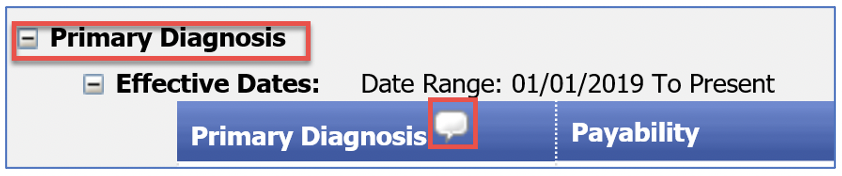
Facility outpatient claims are required to be billed with a HCPCS code. During claims processing, the Primary Diagnosis rule for the HCPCS code is applied to the outpatient facility claim. A provider can submit up to 25 diagnoses on a facility claim.
The information in the new icon indicates the location of the diagnosis on the facility claim that applies to the Primary Diagnosis rule.
When a user hovers over the icon, it will show how the standalone Primary Diagnosis rule is applied to an outpatient facility claim billed with the same HCPCS code.
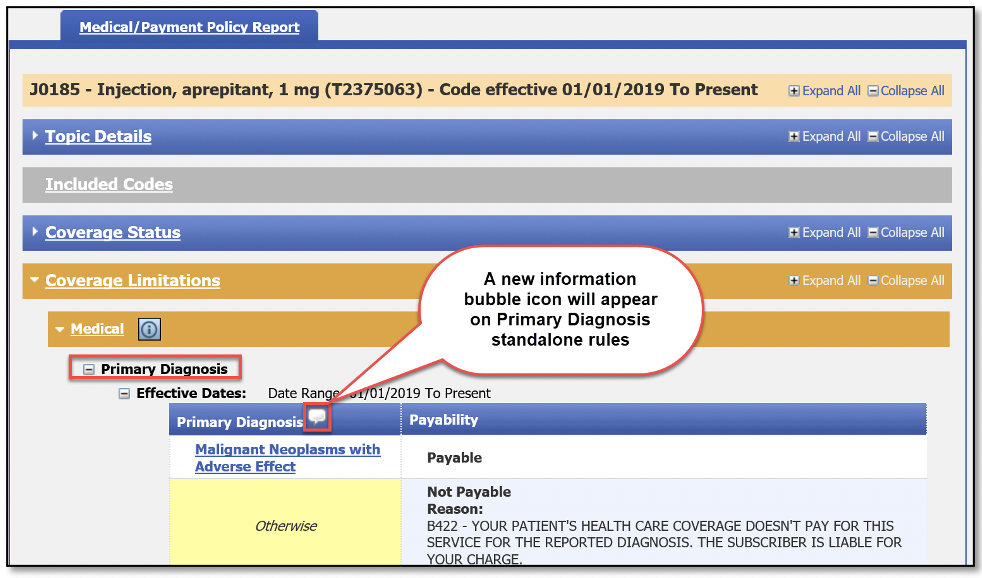
The four types of information that could appear in the information bubble are:
- Primary — The diagnosis restriction on the outpatient facility claim will only be based on the primary diagnosis code billed.
- Primary or Secondary — The diagnosis restriction on the outpatient facility claim will be based on the primary diagnosis or the second diagnosis code billed.
- Any — The diagnosis restriction on the outpatient facility claim will be based on any diagnosis code billed.
- Does not apply — A diagnosis restriction will not be applied to the outpatient facility claim.
The screenshot below is a view of what users can expect to see. The screenshot is of HCPCS code J0185 on the Medical/Payment Policy tab under Coverage Limitations, Medical, Primary Diagnosis.
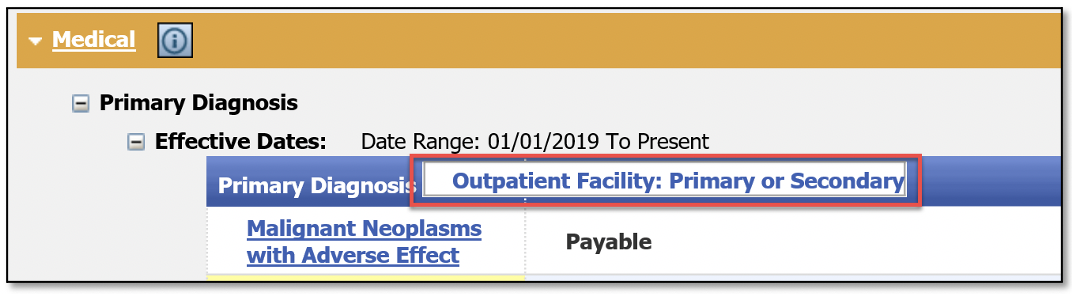
When a user hovers over the icon, the type of diagnosis information will appear.
If you have any questions, contact Provider Inquiry.
Here’s more of what you need to know about the respiratory therapy services billing policy
Blue Cross Blue Shield of Michigan has updated its provider manuals to include a new policy that facilities should use when billing respiratory therapy services. The new policy went into effect Jan. 1, 2021, and we wrote about it in a January 2021 Record article.
We subsequently received some questions from providers about this change. Following is a list of answers to some frequently asked questions, which we hope will make the transition as smooth as possible for health care providers.
Q: Does the new policy pertain to both inpatient and outpatient?
A: The new policy pertains to inpatient only.
Q: Does there need to be a change in coding?
A: No. There will be no changes to revenue codes or units. The only change being made will be in the amount being charged.
Q: Can you give an example of how providers should be billing now for claims with admission dates of Jan. 1, 2021, and later, versus how they billed previously?
A: Yes. Let’s say that on a single day of service, a patient is on the ventilator for five hours and then weaned to CPAP for the remaining 19 hours. Previously, services were billed at a daily rate, regardless of hours used. But with the new policy, providers should be adjusting the charges billed to reflect only the hours used (for example, dividing the daily charge by 24 hours to determine an hourly charge and multiplying by actual hours used).
Q: Can these claims be audited?
A: Yes. Every claim is subject to audit.
Q: Why is Blue Cross making these changes when other payers have not?
A: Blue Cross has the obligation to make sure we pay claims correctly. The new policy supports this effort. We understand this may not have been how things were handled in the past, but industry norms have been shifting. Payers and customers are highly concerned that overpayment of claims is being overlooked and not identified up front. Implementing new, innovative ways to address and prevent overpayments early on will reduce the necessity for a back‑end review and recovery effort for both facilities and Blue Cross.
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
Billing policy and guidelines for intensity modulated radiation therapy
When billing for intensity modulated radiation therapy, or IMRT, the following guidelines should be followed, in accordance with a new Blue Cross Blue Shield of Michigan policy. This policy has been adopted to align billing requirements with industry and Centers for Medicare & Medicaid Services standards.
When an IMRT simulation is performed on the same tumor within 14 days before an IMRT plan, reimbursement of the simulation will be included in the reimbursement whether the simulation is reported on the same or different date of service. In addition, the IMRT policy addresses certain radiation therapy services that may be performed 14 days before, on, or as part of the development of the IMRT plan.
In accordance with the American Medical Association and CMS’ National Correct Coding Initiative Policy Manual, Blue Cross considers CPT codes *77014, *77280, *77285, *77290, *77295, *77306 through *77321, *77331 and *77370 as included in the payment for CPT code *77301 (IMRT planning) when performed in the development of the IMRT plan on the same or different dates of service for the same tumor. To report services for a different tumor on a different date of service, use the appropriate modifier to identify that it is separate, distinct and unrelated to the IMRT plan.
IMRT simulation services billed separately and not billed according to the above guidelines will be denied.
Reminder to update your Provider Authorization form when changes occur
Blue Cross Blue Shield of Michigan is dedicated to safeguarding the protected health information of its members. These safeguards include completion of a Trading Partner Agreement and Provider Authorization form as part of the electronic data interchange setup process. All EDI trading partners must complete a TPA and Provider Authorization form before they can exchange PHI with Blue Cross.
The terms of the TPA require you to notify Blue Cross of any changes in your trading partner information, so it’s important to keep your Provider Authorization form up to date. Updating the form also ensures information is routed to the proper destination.
Update the form when you switch:
- Service bureaus or clearinghouses
- Software vendors
- Billing services
- 835‑file recipients
- Submitter IDs
- Unique 835 receivers or Trading Partner IDs
Review the form when you:
- Join a new group practice
- Leave a group practice and start billing using your own NPI
- Hire a new billing service
- Start submitting claims through a clearinghouse or when you’ve changed clearinghouses
- Decide you no longer want to receive 835 remittance files
- Select a new destination for your 835
You don’t need to update the Provider Authorization form if your submitter and Trading Partner IDs don’t change.
How to change your EDI setup
To make changes to your EDI setup log in to the Trading Partner Agreement webpage.
Or follow the complete navigation steps to arrive at the TPA login page:
- Visit bcbsm.com/providers
- Scroll down to the Provider resources section and click on Quick Links.
- Click on Electronic Connectivity EDI.
- Click on How to use EDI to exchange information with us electronically.
- Scroll down to the EDI agreements section and click on Update your Provider Authorization Form.
If you have any questions about EDI enrollment, contact the EDI Help Desk at 1‑800‑542‑0945. For assistance with the TPA or the Provider Authorization form, select the TPA option.
![]()
Additional drugs covered under Medicare Part B now available at retail pharmacies
Beginning in April, pharmacies can begin billing Medicare Plus Blue℠ plans directly for certain drugs approved for coverage under the Medicare Part B benefit. Previously, many retail pharmacies were unable to bill Part B medications directly and often charged members in full for these drugs. This required members to complete and submit a reimbursement form to Blue Cross Blue Shield of Michigan.
Any existing cost share for these drugs still applies, according to the member’s plan.
The table below lists the medication types and how they will be processed.
| Drug type | What’s new | Medicare Advantage Plan(s) included |
|---|---|---|
| Nebulizer solutions | Will automatically process under Part B if member lives at home and under Part D if member resides in a long-term care or skilled nursing facility |
Medicare Plus Blue℠ plans with or without prescription coverage if Blue Cross ID card used** |
| Select oral cancer medications | Will process under Part B; no prior authorization required |
Medicare Plus Blue℠ plans with or without prescription coverage if Blue Cross ID card used |
| Oral antiemetics and immunosuppressants | Will process under the appropriate benefit once a coverage review of Part B versus Part D is complete |
Medicare Plus Blue℠ plans with prescription coverage*** |
**For members without a Medicare Plus Blue prescription drug plan who reside in a long‑term care or skilled nursing facility, bill using their Part D plan ID card.
***For members without a Medicare Plus Blue prescription drug plan, if the oral antiemetic or immunosuppressant is approved for Part B coverage, bill using the CMS 1500 form.
View the lists of Medicare Part B drugs available at point of service depending on the member’s plan as follows:
Medicare Plus Blue only
Medicare Plus Blue with prescription drug coverage
Exclusions
Prescription Blue℠ PDP members are excluded from this program.
Additional medical benefit drugs to require prior authorization for some commercial members
What you need to know
For dates of service on or after May 24, 2021, you’ll need to submit prior authorization requests to AIM Specialty Health® for Herceptin Hylecta™ (trastuzumab and hyaluronidase‑oysk), Herzuma® (trastuzumab‑pkrb), Ogivri™ (trastuzumab‑dkst) and Trazimera™ (trastuzumab‑qyyp) for some commercial members.
Blue Cross Blue Shield of Michigan will add prior authorization requirements for additional drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust non‑Medicare members.
For dates of service on or after May 24, 2021, submit prior authorization requests to AIM Specialty Health® for these drugs:
- Herceptin Hylecta™ (trastuzumab and hyaluronidase‑oysk), HCPCS code J9356
- Herzuma® (trastuzumab‑pkrb), HCPCS code Q5113
- Ogivri™ (trastuzumab‑dkst), HCPCS code Q5114
- Trazimera™ (trastuzumab‑qyyp), HCPCS code Q5116
How to submit requests
Submit requests through the AIM ProviderPortal** or by calling the AIM Contact Center at 1‑844‑377‑1278.
For information about registering for and accessing the AIM ProviderPortal, see the Frequently Asked Questions page** on the AIM website.
More about the authorization requirements
Authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
For more information on requirements related to drugs covered under the medical benefit for these members, refer to the Medical oncology prior authorization list for UAW Retiree Medical Benefit Trust PPO non‑Medicare members. We’ll update this list to reflect these changes prior to the effective date.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Blue Cross and BCN ending COVID‑19 anti‑stockpiling quantity limits
What you need to know
- Temporary quantity limits on select medications are ending April 1.
- We’re also making changes to quantity limits for albuterol inhalers.
- We’re reminding you of how to submit quantity limit waiver requests.
On April 1, 2021, Blue Cross Blue Shield of Michigan and Blue Care Network will end temporary anti‑stockpiling quantity limits on select medications used to treat COVID‑19 symptoms.
We adopted temporary quantity limits in April 2020 on select medications to prevent unnecessary stockpiling during the COVID‑19 public health crisis and to ensure access for members who need these medications to treat their chronic conditions. This was announced in an April 30, 2020, provider alert.
Quantity limits will return to what they were before the temporary COVID‑19 policy, with the exception of albuterol inhalers. We previously covered four albuterol inhalers per 30 days. Effective April 1, we’ll cover two inhalers per 30 days. This change isn’t related to the COVID‑19 public health crisis. Consult the Temporary quantity limits on some prescription drugs during the COVID‑19 pandemic document for details, including instructions on how to request a quantity limit waiver if you have a patient who needs to exceed the albuterol inhaler limit. Physicians requesting more than two inhalers per month for a patient can follow the usual process for requesting an exception to the quality limits.
For information on changes related to the COVID‑19 pandemic, see the Temporary changes due to the COVID‑19 pandemic document. It’s on our coronavirus webpage, which is available through Provider Secured Services and on our public website at bcbsm.com/coronavirus (click on the Health Care Providers tab).
How to submit quantity limit waiver requests
Physicians can submit quantity limit waiver requests through our standard prior authorization processes.
To request authorization in writing, fax a Medication Request Form — Quantity Limit Request to the Blue Cross Pharmacy Clinical Help Desk at 1‑866‑601‑4425. To find the form:
- Log in to web‑DENIS.
- Click on BCBSM Provider Publications and Resources.
- Click on Commercial Pharmacy Prior Authorization and Step Therapy forms.
- Click on Quantity Limit Request.
If you have any questions, call the Pharmacy Clinical Help Desk at 1‑800‑437‑3803.
![]()
Clarification: Blue Cross has selected Northwood as its DME/POS benefit manager
What you need to know
- Participating in the Northwood network may result in more limited provider options for some members.
- The move to the Northwood network also applies to members who receive supplies or devices directly from the manufacturer.
Effective Jan. 1, 2021, Blue Cross Blue Shield of Michigan selected Northwood Inc., as its durable medical equipment, prosthetics, orthotics and medical supplies benefit manager for Blue Cross Blue Shield of Michigan fully insured commercial and individual members who reside in Michigan.
Affected members were notified about the change by letter or email in October 2020. The notification included information about how to locate a Northwood provider. Members are encouraged to use network providers through the Northwood network to help keep costs as low as possible.
Keep in mind that participating in the Northwood network may result in more limited provider options for some members. They may need to travel farther to obtain a specific brand or device or they may need to find a replacement brand or device not available with their local provider.
We wanted to clarify that this change also applies to members who receive supplies or devices directly from the manufacturer (for example, Medtronic or Dexcom). Currently, there is no process to provide exceptions to the network guidelines. We advise members to always check their benefits when considering an out‑of‑network option.
For more information about the Northwood program and how it works, refer to the Northwood DMEPOS Management Program FAQ and the Northwood DMEPOS Management Program Procedure Code Requiring Prior Authorization documents located on bcbsm.com/providers.
If you have questions about the Northwood DME/POS management program, contact Northwood Provider Relations at 1‑800‑447‑9599 between 8:30 a.m. and 5 p.m. Eastern time Monday through Friday.
No portion of this publication may be copied without the express written permission of Blue Cross Blue Shield of Michigan, except that BCBSM participating health care providers may make copies for their personal use. In no event may any portion of this publication be copied or reprinted and used for commercial purposes by any party other than BCBSM.
*CPT codes, descriptions and two-digit numeric modifiers only are copyright 2020 American Medical Association. All rights reserved.
 When Blue Cross Blue Shield of Michigan and Blue Care Network move to the Availity® provider portal later in 2021, you’ll notice some updated features that will help you find what you need more quickly. Here’s a preview of how you can designate specific resources on Availity as “favorites.”
When Blue Cross Blue Shield of Michigan and Blue Care Network move to the Availity® provider portal later in 2021, you’ll notice some updated features that will help you find what you need more quickly. Here’s a preview of how you can designate specific resources on Availity as “favorites.”