|
June 2023

Michigan Automated Prescription System may not capture all information about a patient’s experience with controlled substances
Health care providers using the Michigan Automated Prescription System should know that the database may not capture information about some controlled substances in a patient’s history.
Substances not captured by MAPS include:
- Controlled substances administered or dispensed to patients from opioid treatment programs, such as methadone and buprenorphine
- Samples of controlled substances provided to a patient
- Controlled substances dispensed by a doctor at a medical institution for a maximum of 48 hours
MAPS is an interactive database used to track controlled substances, schedule II through V, including gabapentin.
MAPS provides clinicians with important information about a patient’s controlled substance prescription history. It can be a valuable tool when considering treatment options and screening patients who may be at risk for abuse or diversion.
For more information on MAPS, visit the MI Automated Prescription System (MAPS)** website.
**Blue Cross Blue Shield of Michigan doesn't own or control this website.
Reminder: Prior authorization changes start June 1
Michigan’s prior authorization law requirements** go into effect on June 1, 2023. These requirements apply to Michigan health care plans and providers whose members or patients have commercial coverage.
One of the requirements of the law is that providers must submit prior authorization requests electronically. If providers can’t submit requests electronically due to temporary technical problems, such as power or internet outages, they can submit via alternate methods, such as phone or fax. For alternate submission methods, see the appropriate page of our ereferrals.bcbsm.com website.
The law also requires health care plans to:
- Provide a list of services that require prior authorization and the clinical criteria used to make determinations on prior authorization requests. On June 1, this information will be available at bcbsm.com/priorauth.
- Make determinations on standard (non-urgent) preservice prior authorization requests within nine days of receiving a request. We’ll do this starting June 1.
We’re updating other communications to reflect these changes.
We previously communicated this information and more about the requirements of the law in our provider newsletters and in the provider alert titled “Update: Prior authorization changes coming in June.”
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
HCPCS 1st-quarter update: New and updated codes
The Centers for Medicare & Medicaid Services has added several new codes as part of its quarterly Health Care Procedure Coding System updates. The codes, effective dates and Blue Cross Blue Shield of Michigan’s coverage decisions are below.
Skin substitutes
Code |
Change |
Coverage comments |
Effective date |
A2019 |
Added |
Not covered |
April 1, 2023 |
A2020 |
Added |
Not covered |
April 1, 2023 |
A2021 |
Added |
Not covered |
April 1, 2023 |
Q4265 |
Added |
Not covered |
April 1, 2023 |
Q4266 |
Added |
Not covered |
April 1, 2023 |
Q4267 |
Added |
Not covered |
April 1, 2023 |
Q4268 |
Added |
Not covered |
April 1, 2023 |
Q4269 |
Added |
Not covered |
April 1, 2023 |
Q4270 |
Added |
Not covered |
April 1, 2023 |
Q4271 |
Added |
Not covered |
April 1, 2023 |
Durable medical equipment/Prosthetics and orthotics/Medical supplies
Code |
Change |
Coverage comments |
Effective date |
A4341 |
Added |
Covered |
April 1, 2023 |
A4342 |
Added |
Covered |
April 1, 2023 |
A4560 |
Added |
Not covered |
April 1, 2023 |
A6590 |
Added |
Covered |
April 1, 2023 |
A6591 |
Added |
Covered |
April 1, 2023 |
A7049 |
Added |
Not covered |
April 1, 2023 |
E0677 |
Added |
Covered |
April 1, 2023 |
E0711 |
Added |
Not covered |
April 1, 2023 |
E1905 |
Added |
Not covered |
April 1, 2023 |
K1035 |
Added |
Not covered |
April 1, 2023 |
L8678 |
Added |
Covered |
April 1, 2023 |
Injections/administrative drug
Code |
Change |
Coverage comments |
Effective date |
C9145 |
Added |
Covered |
April 1, 2023 |
C9146 |
Added |
Covered |
April 1, 2023 |
C9147 |
Added |
Covered |
April 1, 2023 |
C9148 |
Added |
Covered |
April 1, 2023 |
C9149 |
Added |
Covered |
April 1, 2023 |
J0208 |
Added |
Covered |
April 1, 2023 |
J0218 |
Added |
Manual review |
April 1, 2023 |
J0610 |
Delete |
Deleted March 31, 2023 |
March 31, 2023 |
J0611 |
Delete |
Deleted March 31, 2023 |
March 31, 2023 |
J0612 |
Added |
Covered |
April 1, 2023 |
J0613 |
Added |
Covered |
April 1, 2023 |
J1411 |
Added |
Covered |
April 1, 2023 |
J1449 |
Added |
Covered |
April 1, 2023 |
J1747 |
Added |
Covered |
April 1, 2023 |
J2403 |
Added |
Covered |
April 1, 2023 |
J9196 |
Added |
Covered |
April 1, 2023 |
J9294 |
Added |
Covered |
April 1, 2023 |
J9296 |
Added |
Covered |
April 1, 2023 |
J9297 |
Added |
Covered |
April 1, 2023 |
Q5127 |
Added |
Covered |
April 1, 2023 |
Q5128 |
Added |
Covered |
April 1, 2023 |
Q5129 |
Added |
Covered |
April 1, 2023 |
Q5130 |
Added |
Covered |
April 1, 2023 |
S9563 |
Added |
Not covered |
April 1, 2023 |
Modifiers
Code |
Change |
Coverage comments |
Effective date |
JK |
Added |
Informational only |
April 1, 2023 |
JL |
Added |
Informational only |
April 1, 2023 |
Data gathering
Code |
Change |
Coverage comments |
Effective date |
M0010 |
Added |
Provider liable |
April 1, 2023 |
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
HCPCS replacement codes, effective April 1, 2023, established
Q5127 replaces C9399, J3490, J3590 and J9999 when billing for Stimufend (pegfilgrastim-fpgk), a biosimilar
The Centers for Medicare & Medicaid Services has established a permanent procedure code for specialty medical drug Stimufend (pegfilgrastim-fpgk), a biosimilar.
All services through March 31, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2023, must be reported with Q5127.
Prior authorization is required through the Medical Benefit Drug program for Q5127 for all groups unless they’re opted out of the program.
For groups that have opted out of the prior authorization program, this code is covered for its FDA-approved indications.
Q5128 replaces C9399, J3490, J3590 and J9999 when billing for Cimerli (ranibizumab-eqrn), a biosimilar
CMS has established a permanent procedure code for specialty medical drug Cimerli (ranibizumab-eqrn), a biosimilar.
All services through March 31, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2023, must be reported with Q5128.
Prior authorization is required through the Medical Benefit Drug program for Q5128 for all groups unless they’re opted out of the program.
For groups that have opted out of the prior authorization program, this code is covered for its FDA-approved indications.
Q5129 replaces C9399, J3490, J3590 and J9999 when billing for Vegzelma (bevacizumab-adcd), a biosimilar
CMS has established a permanent procedure code for specialty medical drug Vegzelma (bevacizumab-adcd), a biosimilar.
All services through March 31, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2023, must be reported with Q5129.
Prior authorization is required through the Medical Benefit Drug program for Q5129 for all groups unless they’re opted out of the program.
For groups that have opted out of the prior authorization program, this code is covered for its FDA-approved indications.
Q5130 replaces C9399, J3490, J3590 and J9999 when billing for Fylnetra (pegfilgrastim-pbbk), a biosimilar
CMS has established a permanent procedure code for specialty medical drug Fylnetra (pegfilgrastim-pbbk), a biosimilar.
All services through March 31, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2023, must be reported with Q5130.
Prior authorization is required through the Medical Benefit Drug program for Q5130 for all groups unless they’re opted out of the program.
For groups that have opted out of the prior authorization program, this code is covered for its FDA-approved indications.
C9145 replaces J3490, J3590 when billing for Aponvie (aprepitant) for facility services only
CMS has established a permanent procedure code for specialty medical drug Aponvie (aprepitant).
All services through March 31, 2023, will continue to be reported with code J3490 and J3590. All facility services performed on and after April 1, 2023, must be reported with C9145, while all professional services will continue to be reported with J3490 and J3590.
C9146 replaces J9999 when billing for Elahere (mirvetuximab soravtansine-gynx) for facility services only
CMS has established a permanent procedure code for specialty medical drug Elahere.
All services through March 31, 2023, will continue to be reported with J9999. All facility services performed on and after April 1, 2023, must be reported with C9146, while all professional services will continue to be reported with J9999.
C9147 replaces J9999 when billing for Imjudo (tremelimumab-actl) for facility services only
CMS has established a permanent procedure code for specialty medical drug Imjudo.
All services through March 31, 2023, will continue to be reported with J9999. All facility services performed on and after April 1, 2023, must be reported with C9147, while all professional services will continue to be reported with J9999.
C9148 replaces J3590 when billing for Tzield (teplizumab-mzwv) for facility services only
CMS has established a permanent procedure code for specialty medical drug Tzield.
All services through March 31, 2023, will continue to be reported with J3590. All facility services performed on and after April 1, 2023, must be reported with C9148, while all professional services will continue to be reported with J3590.
C9149 replaces C9399, J3490 and J3590, and when billing for Tzield (teplizumab-mzwv) for facility services only
CMS has established a permanent procedure code for specialty medical drug Tzield.
All services through March 31, 2023, will continue to be reported with J3590. All facility services performed on and after April 1, 2023, must be reported with C9149, while all professional services will continue to be reported with J3590.
J0208 replaces J3490 when billing for Pedmark (sodium thiosulfate)
CMS has established a permanent procedure code for specialty medical drug Pedmark.
All services through March 31, 2023, will continue to be reported with J3490. All services performed on and after April 1, 2023, must be reported with J0208.
J0218 replaces J3490, J3590 and C9399 when billing for Xenpozyme (olipudase alfa-rpcp)
CMS has established a permanent procedure code for specialty medical drug Xenpozyme.
All services through March 31, 2023, will continue to be reported with J3490, J3590 and C9399. All services performed on and after April 1, 2023, must be reported with J0218.
Site-of-care prior authorization is required through the Medical Benefit Drug program for J0218 for all groups unless they’re opted out of the program.
For groups that have opted out of the Medical Benefit Drug program, this code is covered for its FDA-approved indications and requires manual review.
J1411 replaces C9399, J3490, J3590 and J9999 when billing for Hemgenix (etranacogene dezaparvovec-drlb)
CMS has established a permanent procedure code for specialty medical drug Hemgenix (etranacogene dezaparvovec-drlb), a gene/cellular therapy.
All services through March 31, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2023, must be reported with J1411.
Prior authorization is required through the Medical Benefit Drug program for J1411 for all groups unless they’re opted out of the program.
For groups that have opted out of the Medical Benefit Drug program, this code is covered for its FDA-approved indications and requires manual review.
J1449 replaces J3490, J3590, C9399 and J9999 when billing for Rolvedon (eflapegrastim-xnst)
CMS has established a permanent procedure code for specialty medical drug Rolvedon (eflapegrastim-xnst).
All services through March 31, 2023, will continue to be reported with codes J3490, J3590, C9399 and J9999. All services performed on and after April 1, 2023, must be reported with J1449.
Prior authorization is required through the Medical Benefit Drug program for J1449 for all groups unless they’re opted out of the program.
For groups that have opted out of the prior authorization program, this code is covered for its FDA-approved indications.
J1747 replaces J3490, J3590, C9399 and J9999 when billing for Spevigo (spesolimab-sbzo)
CMS has established a permanent procedure code for specialty medical drug Spevigo (spesolimab-sbzo).
All services through March 31, 2023, will continue to be reported with codes J3490, J3590, C9399 and J9999. All services performed on and after April 1, 2023, must be reported with J1747.
Site-of-care prior authorization is required through the Medical Benefit Drug program for J1747 for all groups unless they’re opted out of the program.
For groups that have opted out of the Medical Benefit Drug program, this code is covered for its FDA-approved indications and requires manual review.
Billing chart: Blue Cross highlights medical, benefit policy changes
You’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart.
This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures.
We'll publish information about new Blue Cross groups or changes to group benefits under the Group Benefit Changes heading.
For more detailed descriptions of the Blue Cross' policies for these procedures, check under the Commercial Policy tab in Benefit Explainer on Availity®. To access this online information:
1. Log in to availity.com.
2 .Click on Payer Spaces on the Availity menu bar.
3. Click on the BCBSM and BCN logo.
4. Click on Benefit Explainer on the Applications tab.
5. Click on the Commercial Policy tab.
6. Click on Topic.
7. Under Topic Criteria, click on the circle for Unique Identifier and click the drop-down arrow next to Choose Identifier Type, then click on HCPCS Code.
8. Enter the procedure code.
9. Click on Finish.
10. Click on Search.
| Code* |
BCBSM changes to:
Basic Benefit and Medical Policy, Group
Variations Payment Policy, Guidelines
|
| POLICY CLARIFICATIONS |
11920,*** 11921,*** 11922,*** 17380,*** 19325,*** 21120,*** 21121,*** 21122,*** 21123,*** 21125,*** 21127,*** 21137,*** 21138,*** 21209,*** 30400,*** 30410,*** 30420, 15769, 15771, 15772, 15773, 15774, 17999,** 19303, 19318, 19350, 31599,** 31899,** 54520, 55970, 55980, 56805, 57291, 57292, 57335, 58150, 58152, 58180, 58260, 58262, 58275, 58291, 58541, 58542, 58543, 58544, 58550, 58552, 58553, 58554
Diagnosis codes
F64.0, F64.8, F64.9, Z87.890
**Unlisted codes
***Payable with listed diagnoses
Experimental/non-payable
11950, 11951, 11952, 11954, 15769, 15771, 15772, 15773, 15774, 15820, 15821, 15822, 15823, 15824, 15825, 15826, 15828, 15830, 15832, 15833, 15834, 15835, 15836, 15837, 15838, 15839, 15876, 15877, 15878, 15879, 19316, 21208, 30430, 30435, 30450, 69300, Q2026, Q2028 |
Basic benefit and medical policy
Gender affirming services
The gender affirming services policy has been updated to cover additional procedure codes, update established codes with additional payable diagnoses and add non-payable diagnoses for specific procedure codes when criteria are met, effective Jan. 1, 2023.
The safety and effectiveness of select medical and surgical treatments for gender dysphoria have been established. The established treatments for gender dysphoria include:
- Puberty suppression in adolescents
- Hormone therapy (for masculinization/feminization) for adolescents who meet criteria and adults
- Medically necessary gender affirming surgery:**
- Genitalia reconstruction
- Mastectomy for a transgender man (a person who has a gender identity as a man and who was assigned female at birth)
- Augmentation mammoplasty (implants) for transgender women (a person who has a gender identity as a woman and who was assigned male at birth)
- Thyroid reduction chondroplasty (tracheal shave)
- Facial feminization
- Facial masculinization
**Gender-affirming surgery may require prior authorization.
Gender-specific services may be medically necessary for transgender and gender-diverse people appropriate to their anatomy. Examples include:
- Breast cancer screening in transgender and gender-diverse people with breasts from natal puberty who haven’t undergone gender-affirming chest surgery and for transgender and gender diverse people who have received estrogens, taking into account the length of time of hormone use, dosing, current age and age at which the hormones were initiated
- Prostate cancer screening for transgender and gender diverse people who have retained their prostate
- Cervical screening for transgender and gender diverse people who currently have or previously had a cervix following local guidelines for cisgender women.
- Obvstetric services for transgender and gender diverse people when they are pregnant
- To guide preventive medical care, any anatomical structure present that warrants screening should be screened, regardless of gender identity.
Inclusions:
Gender-affirming surgery may be indicated for members who meet all the following inclusionary criteria:
- Chest surgery***
- Mastectomy is considered reconstructive when all the following criteria have been met:
- The individual is at least 18 years of age.
- The individual has been diagnosed with gender dysphoria (see Description/Background section for diagnostic criteria).
- Gender dysphoria is marked and sustained.
- The individual has capacity to make fully informed decisions and consent for treatment.
- One letter of assessment as indicated below****
- Other possible causes of apparent gender incongruence have been identified and excluded.
- Mental health and physical conditions that could negatively affect the outcome of gender-affirming medical treatments have been assessed, with the risks and benefits discussed, before a decision is made regarding treatment.
- Hormone therapy prior to mastectomy isn’t required, as the aim of hormone therapy before facial surgery or gonadectomy is primarily to introduce a period of reversible testosterone or estrogen suppression before the individual undergoes irreversible surgical intervention.
- Living in a gender role congruent with gender identity for 12 continuous months isn’t required prior to a mastectomy.
- Pre-operative and post-operative care that addresses both surgical results and possible behavioral health results is highly recommended.
Nipple reconstruction, including tattooing, following a gender affirming mastectomy that meets the reconstructive criteria above is considered reconstructive.
- Breast augmentation is considered reconstructive when all the following criteria have been met:
- The individual is at least 18 years of age.
- The individual has been diagnosed with gender dysphoria (see Description/Background section for diagnostic criteria).
- Gender dysphoria is marked and sustained.
- The individual has capacity to make fully informed decisions and consent for treatment.
- One letter of assessment as indicated below****
- Other possible causes of apparent gender incongruence have been identified and excluded.
- Mental health and physical conditions that could negatively affect the outcome of gender-affirming medical treatments have been assessed, with the risks and benefits discussed, before a decision is made regarding treatment.
- The individual is stable on their gender-affirming hormonal treatment regimen for at least 12 months, unless a rationale is provided by the HCP that indicates that hormone treatment is either contraindicated or not necessary for the individual’s clinical situation.
- Living in a gender role congruent with gender identity for 12 continuous months isn’t required prior to breast augmentation.
- Existing chest appearance demonstrates significant variation from normal appearance for the experienced gender.
- Pre-operative and post-operative care that addresses both surgical results and possible behavioral health results is highly recommended.
***The procedures needed to reconstruct a feminine/masculine appearance can only be performed once per lifetime.
- Facial surgery***† is considered reconstructive when all the following criteria have been met:
- The individual is at least 18 years of age.
- The individual has been diagnosed with gender dysphoria (see Description/Background section for diagnostic criteria).
- Gender dysphoria is marked and sustained.
- The individual has capacity to make fully informed decisions and consent for treatment.
- Other possible causes of apparent gender incongruence have been identified and excluded.
- One letter of assessment as indicated below****
- Mental health and physical conditions that could negatively affect the outcome of gender-affirming medical treatments have been assessed, with the risks and benefits discussed, before a decision is made regarding treatment.
- The individual is stable on their gender-affirming hormonal treatment regimen for at least 12 months, unless a rationale is provided by the HCP that indicates that hormone treatment is either contraindicated or not necessary for the individual’s clinical situation.
- The new gender identity should be present for at least 12 months.
- The member has a consistent stable gender identity that is well documented by their treating providers and, when possible, lives as their affirmed gender in places where it is safe to do so.
- Existing facial appearance demonstrates significant variation from normal appearance for the experienced gender.
- The procedure directly addresses variation from normal appearance for the experienced gender (Note: Each procedure requested should be considered separately as some procedures may be cosmetic and others may be reconstructive).
- Pre-operative and post-operative care that addresses both surgical results and possible behavioral health results is highly recommended.
***The procedures needed to reconstruct a feminine/masculine appearance can only be performed once per lifetime.
†List of procedures included in this group is thyroid reduction chondroplasty (tracheal shave), genioplasty (repositioning or reshaping of the chin), mandible augmentation (jawline contouring/reconstruction), facial bone reduction, forehead reduction/contouring, rhinoplasty (reshaping/contouring of the nose).
- Genital surgery is considered medically necessary when all the following criteria have been met:
- The individual is at least 18 years of age.
- The individual has been diagnosed with gender dysphoria (see Description/Background section for diagnostic criteria).
- Gender dysphoria is marked and sustained.
- The individual has capacity to make fully informed decisions and consent for treatment.
- Other possible causes of apparent gender incongruence have been identified and excluded.
- One letter of assessment as indicated below****
- Mental health and physical conditions that could negatively affect the outcome of gender-affirming medical treatments have been assessed, with the risks and benefits discussed, before a decision is made regarding treatment.
- The individual is stable on their gender affirming hormonal treatment regimen for at least 12 months, unless a rationale is provided by the HCP that indicates that hormone treatment is either contraindicated or not necessary for the individual’s clinical situation.
- The new gender identity should be present for at least 12 months.
- The member has a consistent stable gender identity that is well documented by their treating providers and, when possible, lives as their affirmed gender in places where it is safe to do so.
- Pre-operative and post-operative care that addresses both surgical results and possible behavioral health results is highly recommended.
These criteria don’t apply to patients who are having these surgical procedures for medical indications other than gender dysphoria.
****Letter requirements:
- Required for hormone therapy for adolescents
- Required for facial, pelvic, gonadal and/or genital surgery for adults
Adolescents
One letter of assessment from the multidisciplinary team (or in situations where a multidisciplinary team isn’t available, a professional from one of the multiple disciplines who are experts in transgender health and in the management of the care required for transgender and gender-diverse adolescents who is taking care of the individual) is required for adolescents receiving gender-affirming medical treatment.
This letter needs to reflect the assessment and opinion from the team, which involves both medical HCPs and mental health professionals, supporting that the individual meets the criteria for gender dysphoria. The detailed assessment must have been performed within 12 months of the requested submission.
The HCP assessing and working with the adolescent should meet all the following criteria:
- Are licensed by their professional body and hold a postgraduate degree or its equivalent in a clinical field related to transgender health granted by a nationally accredited institution.
- Receive theoretical and evidenced-based training and have expertise in general child, adolescent and family mental health across the developmental spectrum.
- Receive training and have expertise in gender identity development, gender diversity in children and adolescents, have the ability to assess capacity to assent/consent and possess general knowledge of gender diversity across the lifespan.
- Continue engaging in professional development in all areas relevant to gender-diverse children, adolescents and families.
Adults
One letter of assessment from an HCP who has competencies in the assessment of transgender and gender diverse people, documenting that the individual meets the criteria for gender dysphoria, is required for transgender and gender diverse adults who meet the below criteria for gender-affirming medical and surgical treatments. The detailed assessment must have been performed within 12 months of the requested submission.
The HCP should meet all the following criteria:
- Are licensed by their professional body and hold, at a minimum, a master’s degree (or equivalent training in a clinical field related to transgender health or equivalent further clinical training in this area) that’s granted by a nationally accredited institution
- Should be competent using the latest edition of the Diagnostic and Statistical Manual of Mental Disorders, or DSM-5, for diagnosis
- Are able to identify co-existing mental health or other psychosocial concerns and distinguish these from gender dysphoria, incongruence and diversity
- Are able to assess capacity to consent for treatment
- Have experience or be qualified to assess clinical aspects of gender dysphoria, incongruence and diversity
- Undergo continuing education in health care relating to gender dysphoria, incongruence and diversity.
Exclusions:
- Transgender services aren’t covered if contract or certificate language contains specific exclusion of these services.
- Reversal of transgender surgical procedures.
- All procedures that are primarily cosmetic and not reconstructive or not medically necessary including, but not limited to:
- Abdominoplasty
- Blepharoplasty
- Brow lift
- Calf implants
- Cheek/malar implants
- Chin/nose implants
- Collagen injections
- Drugs for hair loss or growth
- Forehead lift
- Hair removal (for exception: see Inclusions, Electrolysis)
- Hair transplantation
- Injectable dermal fillers (i.e. Sculptra, Radiesse)
- Lip reduction
- Liposuction
- Mastopexy
- Neck tightening
- Otoplasty
- Pectoral implants
- Removal of redundant skin
- Rhytidectomy
- Speech-language therapy
|
76376, 76377, 76641, 76642 |
Basic benefit and medical policy
Ultrasound for breast cancer screening
An update to the medical policy statement was made for ultrasound for breast cancer screening, effective May 1, 2023.
Medical policy statement:
Ultrasound imaging of the breast for breast cancer screening hasn’t been shown to be an effective alternative to mammogram alone. Therefore, the use of ultrasound imaging for routine breast cancer screening is experimental.
|
80305-80307, G0480-G0483, G0659
Not medically necessary/not covered:
80320-80377, 83992 |
Basic benefit and medical policy
Drug testing in pain management and substance use disorder treatment
Presumptive and definitive drug testing in the outpatient setting may be considered established when criteria are met.
Inclusionary criteria have been updated, effective May 1, 2023.
Note: This policy addresses drug testing in an outpatient setting. The policy doesn’t apply to drug testing in emergency department, acute inpatient medical or behavioral health facility settings, or testing ordered by or on behalf of a health care provider or facility that receives per-diem reimbursement that includes clinical diagnostic laboratory testing (skilled nursing facility).
Inclusions:
- Presumptive drug testing
- For outpatient pain management, presumptive drug testing is considered established in:
- Baseline screening at the initiation of treatment
- Subsequent monitoring of treatment at an appropriate frequency based on the risk level of the individual, including assessment of aberrant behavior
- For outpatient substance use disorder treatment, presumptive drug testing is considered established in:
- Baseline screening at the initiation of treatment
- Subsequent screening is based on the risk level of the individual and the substance being used
- For an individual not participating in outpatient pain management or outpatient substance use disorder treatment:
- When a clinical evaluation suggests use of non-prescribed medications, illegal or other substances
- When testing for drug or alcohol exposure during pregnancy
- To rule out a fetal withdrawal syndrome by testing the mother for drug use
- Definitive/confirmatory drug testing
- Definitive drug testing is considered established for one of the following:
- When immunoassays for the relevant drugs are not commercially available
- In situations where definitive drug levels are required for clinical decision making (e.g., unexpected positive test that is inadequately explained by the patient, unexpected negative test, quantitative levels are needed to determine clinical treatment)
Exclusions:
- Drug testing as a third-party requirement (e.g., for employment, licensing, court order)
- Simultaneously testing for the same drug with two specimens from different sources (e.g., blood and urine)
Policy guidelines:
One presumptive and one definitive test code may be billed per date of service.
Billing guidelines for definitive drug testing:
Bill G0480-G0483 and G0659 as appropriate, for the number of drug classes tested.
Bill *80XXX and *83992 to report the appropriate drug or metabolite testing. The codes are no longer individually reimbursed for the purpose of this policy; however, we request that they be reported with the appropriate G code.
Contracted laboratories:
Providers should select contracted laboratories for the processing of drug tests. Referring a member to a non-participating laboratory may result in unnecessary services (such as processing tests not originally ordered) and greater financial liability for the member. The referring provider may be held accountable for any inappropriate behavior on the part of the non-participating laboratory. |
81408, 81445, 81479**
**Not otherwise classified procedure |
Basic benefit and medical policy
Germline variations associated with high breast cancer risk
The safety and effectiveness of testing for ATM, CDH1, BARD1, CHEK2, NFI, PTEN, RAD51C, RAD51D, STK11 and TP53 variants for breast cancer risk assessment in adults is considered established. It may be considered a useful diagnostic option when indicated.
Inclusionary criteria have been updated, effective May 1, 2023.
Inclusions:
Criteria for genetic risk evaluation
The National Comprehensive Cancer Network, or NCCN, provides criteria for genetic risk evaluation for individuals with no history or breast cancer and for those with a breast cancer. Updated versions of the criteria are available on the NCCN website.**
Notes:
- For the purpose of this policy, close blood relatives include first-, second- and third-degree relatives who are blood relatives on the same side of the family (maternal or paternal), such as:
- First-degree relatives, who are parents, siblings and children
- Second-degree relatives, who are grandparents, aunts, uncles, nieces, nephews, grandchildren and half-siblings
- Third-degree relatives, who are great-grandparents, great-aunts, great-uncles, great-grandchildren and first cousins
- For the purpose of this policy, high-risk and very-high-risk prostate cancer groups are defined as follows:
- High-risk group: No very-high-risk features and are T3a (American Joint Committee on Cancer staging T3a=tumor has extended outside of the prostate but has not spread to the seminal vesicles); or Grade Group 4 or 5; or prostate specific antigen of 20 ng/ml or greater.
- Very-high-risk group: T3b-T4 (tumor invades seminal vesicles; or tumor is fixed or invades adjacent structures other than seminal vesicles such as external sphincter, rectum, bladder, levator muscles or pelvic wall); or Primary Gleason Pattern 5; or two or three high-risk features; or greater than 4 cores with Grade Group 4 or 5.
Inclusions:
Testing is clinically indicated in the following scenarios:
- Individuals with any close blood relative with a known ATM, CDH1, BARD1, CHEK2, NFI, PTEN, RAD51C, RAD51D, STK11 or TP53 pathogenic/likely pathogenic variant
- Individuals meeting the criteria below but with previous limited testing (e.g., single gene and/or absent deletion duplication analysis) who are interested in multi-gene testing
- Genetic testing for individuals with RAD51C and RAD51D variants who have a personal history of epithelial ovarian cancer (including fallopian tube cancer or peritoneal cancer) at any age
- Genetic testing for ATM, CDH1, BARD1, CHEK2, NFI, PTEN, RAD51C, RAD51D, STK11 or TP53 variants in cancer-affected individuals may be considered appropriate under any of the following circumstances:
- Personal history of breast cancer, including invasive and ductal carcinoma in situ breast cancers, and any of the following:
- Diagnosed age ≤ 50 years
- Diagnosed at any age with any of the following:
- Pathology/histology (any of the following):
- Triple-negative breast cancer
- Multiple primary breast cancers (synchronous or metachronous)
- Lobular breast cancer with personal or family history of diffuse gastric cancer
- Male breast cancer
- Ancestry: Ashkenazi Jewish ancestry
- Family history of any of the following:
- ≥1 close blood relative with any of the following:
- Breast cancer diagnosed ≤50 years
- Male breast cancer any age
- Ovarian cancer any age
- Prostate cancer with metastatic, or high- or very high-risk group any age
- Pancreatic cancer any age
- ≥2 close blood relatives with breast or prostate cancer (any grade) at any age
- ≥3 total diagnoses of breast cancer in patient or close blood relative
- Genetic testing for ATM, CDH1, BARD1, CHEK2, NFI, PTEN, RAD51C, RAD51D, STK11 or TP53 variants of cancer-unaffected individuals may be appropriate under the following circumstances:
- An affected individual not meeting the criteria above or unaffected individual with a first- or second-degree blood relative meeting any of the criteria listed above under “cancer-affected individuals”
- If the affected relative has pancreatic cancer or prostate cancer, only first-degree relatives should be offered testing unless indicated based on additional family history.
Exclusions:
- Patients not meeting any of the above criteria
- Genetic testing for ATM, CDH1, BARD1, CHEK2, NFI, PTEN, RAD51C, RAD51D, STK11 or TP53 variants in minors
Note: For BRCA1/2 and PALB2 testing, refer to policy “Germline Genetic Testing for BRACA1, BRACA2 and PALB2 for Hereditary Breast Ovarian Cancer Syndrome.”
**Blue Cross Blue Shield of Michigan doesn't own or control this website. |
J3490,
J3590 |
Basic benefit and medical policy
Daxxify (daxibotulinumotoxina-lanm)
Daxxify (daxibotulinumotoxina-lanm) is considered established when criteria are met, effective Sept. 7, 2022.
Daxxify (daxibotulinumtoxinA-lanm) is an acetylcholine release inhibitor and neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator or procerus muscle activity in adult patients.
Dosage and administration:
Glabellar lines: 0.1 mL (8 units) by intramuscular injection into each of five sites, for a total dose of 40 units.
Dosage forms and strengths:
For injection: 50 units or 100 units sterile lyophilized powder in a single-dose vial
Daxxify (daxibotulinumotoxina-lanm) isn’t a benefit for URMBT. |
J3490,
J3590 |
Basic benefit and medical policy
Hemgenix (etranacogene dezaparvovec-drlb)
Hemgenix (etranacogene dezaparvovec-drlb) is payable for its FDA-approved indications, effective Nov. 22, 2022.
Hemgenix is an adeno-associated virus vector-based gene therapy indicated for the treatment of adults with hemophilia B (congenital Factor IX deficiency) with one of the following:
- Currently use Factor IX prophylaxis therapy
- Have current or historical life-threatening hemorrhage
- Have repeated, serious spontaneous bleeding episodes
Dosage and administration:
For single-use intravenous infusion only.
- Perform baseline testing to select patients, including testing for Factor IX inhibitor presence and liver health tests.
- The recommended dose of Hemgenix is 2 x 1013 genome copies (gc) per kg of body weight.
- Administer Hemgenix as an intravenous infusion after dilution with 0.9% normal saline at a constant infusion rate of 500 ml/hour (8 mL/min).
Dosage forms and strengths:
Hemgenix is a suspension for intravenous infusion.
Hemgenix is provided in kits containing 10 to 48 single-use vials, each kit constituting a dosage unit based on the patient’s body weight.
Hemgenix has a nominal concentration of 1 x 1013 gc/mL, and each vial contains an extractable volume of not less than 10 mL.
Hemgenix (etranacogene dezaparvovec-drlb) isn’t a benefit for URMBT. |
J3490,
J3590 |
Basic benefit and medical policy
NexoBrid (anacaulase-bcdb)
Effective Dec. 28, 2022, NexoBrid (anacaulase-bcdb) is covered for the following FDA-approved indications:
NexoBrid contains proteolytic enzymes and is indicated for eschar removal in adults with deep partial thickness or full thickness thermal burns.
Limitations of use:
The safety and effectiveness of NexoBrid haven’t been established for treatment of:
- Chemical or electrical burns
- Burns on the face, perineum or genitalia
- Burns on the feet of patients with diabetes mellitus or on the feet of patients with occlusive vascular disease
- Circumferential burns
- Burns in patients with significant cardiopulmonary disease, including inhalation injury
NexoBrid isn’t recommended for wounds contaminated with radioactive and other hazardous substances to avoid unforeseeable reactions with the product and an increased risk of spreading the noxious substance.
Dosage and administration:
- For topical use only.
- NexoBrid may be applied in up to two applications of four hours each.
- A first application may be applied to an area of up to 15% body surface area, or BSA.
- A second application may be applied 24 hours later. The total treated area for both applications must not exceed 20% BSA.
- Use 1.94 g of anacaulase-bcdb in 2 g powder mixed with 20 g gel per 1% BSA, or 4.85 g of anacaulase-bcdb in 5 g powder mixed with 50 g gel per 2.5% BSA.
- Prepare NexoBrid at patient’s bedside within 15 minutes of intended application.
- Apply NexoBrid to a clean, moist wound bed free of burned epidermis layer and blisters, and cover with an occlusive film dressing for four hours.
Dosage forms and strengths:
NexoBrid (anacaulase-bcdb) isn’t a benefit for URMBT.
|
J3490,
J3590 |
Basic benefit and medical policy
Rebyota (fecal microbiota, live – jslm)
Rebyota (fecal microbiota, live – jslm) is considered established when criteria are met, effective Nov. 1, 2022.
Rebyota (fecal microbiota, live – jslm) is indicated for the prevention of recurrence of Clostridioides difficile infection, or CDI, in individuals 18 years of age and older following antibiotic treatment for recurrent CDI.
Limitation of use:
Rebyota (fecal microbiota, live – jslm) isn’t indicated for treatment of CDI.
Dosage and administration:
- For rectal administration only.
- Administer Rebyota (fecal microbiota, live – jslm) 24 to 72 hours after the last dose of antibiotics for CDI.
- Administer a single dose of 150 mL rectally of Rebyota (fecal microbiota, live – jslm).
Dosage forms and strengths:
- Suspension. A single dose is 150 mL.
Rebyota (fecal microbiota, live – jslm) isn’t a benefit for URMBT.
|
J3490,
J3590 |
Basic benefit and medical policy
Sezaby (phenobarbital sodium)
Effective Nov.17, 2022, Sezaby (phenobarbital sodium) is covered for the following FDA-approved indications:
Sezaby is a barbiturate indicated for the treatment of neonatal seizures in term and preterm infants.
Dosage and administration:
- Loading dose: 20 mg/kg is administered by intravenous infusion over 15 minutes into a large peripheral vein. If clinically indicated, at least 15 minutes after completion of the initial loading dose, a second loading dose may be administered over the subsequent 15 minutes as 20 mg/kg for term infants or 10 mg/kg or 20 mg/kg for preterm infants. The maximum total loading dose is 40 mg/kg.
- Maintenance dosage: Starting 8 to 12 hours after first loading dose: 4.5 mg/kg/day given in 2 or 3 divided doses (e.g., 1.5 mg/kg every eight hours or 2.25 mg/kg every 12 hours) up to five days.
- Must be reconstituted with 10 mL 0.9 % sodium chloride Injection, USP prior to administration.
Dosage forms and strengths:
For injection: 100 mg of phenobarbital sodium lyophilized powder in a single-dose vial for reconstitution
Sezaby (phenobarbital sodium) isn’t a benefit for URMBT. |
J3590**
**Not otherwise classified code |
Basic benefit and medical policy
Cosmetic and reconstructive surgery
The cosmetic and reconstructive surgery policy has been updated to state that fat grafting (Renuva™) is considered a cosmetic service, effective May 1, 2023. |
J7297 |
Basic benefit and medical policy
Liletta (levonorgestrel-releasing intrauterine system)
Effective Nov. 10, 2022, Liletta (levonorgestrel-releasing intrauterine system) is covered for the following FDA-approved indications:
Liletta is a progestin-containing intrauterine system indicated for prevention of pregnancy for up to eight years.
Dosage and administration:
- The initial release rate of levonorgestrel is approximately 20 mcg/day and declines progressively to approximately 6.5 mcg/day after eight years; Liletta can be removed at any time but must be removed by the end of the eighth year.
- To be inserted into the uterine cavity with the provided inserter by a trained health care professional using strict aseptic technique. Follow insertion instructions exactly as described.
Re-examination and evaluation should be considered 4 to 6 weeks after insertion and during routine care, or more often if clinically indicated. |
J9119 |
Basic benefit and medical policy
Libtayo (cemiplimab-rwlc)
The FDA has updated the approved indications for Libtayo (cemiplimab-rwlc), effective Nov. 8, 2022. The payable indications include the following:
Libtayo (cemiplimab-rwlc) is a programmed death receptor-1 (PD-1) blocking antibody indicated:
Non-small cell lung cancer
- In combination with platinum‐based chemotherapy for the first‐line treatment of adult patients with non-small cell lung cancer, or NSCLC, with no EGFR, ALK or ROS1 aberrations and is locally advanced where patients are not candidates for surgical resection or definitive chemoradiation or metastatic.
As single agent for the first-line treatment of adult patients with NSCLC whose tumors have high PD-L1 expression (tumor proportion score ≥ 50%) as determined by an FDA-approved test, with no EGFR, ALK or ROS1 aberrations and is locally advanced where patients aren’t candidates for surgical resection or definitive chemoradiation or metastatic. |
| EXPERIMENTAL PROCEDURES |
36836, 36837 |
Basic benefit and medical policy
Endovascular percutaneous device
The use of an endovascular percutaneous device for the creation of an arteriovenous fistula, or pAVF, for hemodialysis access is considered experimental, effective May 1, 2023. |
A9291 |
Basic benefit and medical policy
Prescription digital therapy
Prescription digital therapy is considered experimental for the treatment of attention-deficit/hyperactivity disorder, effective May 1, 2023. |
K1016, K1017 |
Basic benefit and medical policy
eTNS for ADHD
External trigeminal nerve stimulation, or eTNS, for the management of attention deficit hyperactivity disorder is considered experimental (e.g., MonarchM® eTNS System). There is insufficient evidence in the peer-reviewed medical literature to determine the effects of the technology on health outcomes, effective May 1, 2023. |
None of the information included in this billing chart is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.

You’re invited to join PGIP as a physician organization
Blue Cross Blue Shield of Michigan will be accepting applications from new physician organizations for the Physician Group Incentive Program from June 1 through July 31, 2023. To request application materials, send an email to valuepartnerships@bcbsm.com.
About PGIP
The Physician Group Incentive Program was developed with input from providers across Michigan to help improve the quality and efficiency of health care in the state. PGIP facilitates change through a wide range of initiatives, including our nationally recognized Patient-Centered Medical Home program.
Through PGIP, we reward physician organizations for improving health care delivery to their attributed patient population. PGIP-participating physicians are eligible for value-based reimbursement consideration as a result of program efforts.
About physician organizations
A PGIP physician organization consists of physicians participating in our PPO or Traditional network, working together to:
- Transform systems of care to effectively manage patient populations.
- Build the infrastructure needed to optimize, measure and monitor quality of care.
- Promote collaborative relationships.
- Support the most cost-effective delivery of services to improve patient outcomes.
To learn more
- For more information on PGIP and its initiatives, visit the Physician Group Incentive Program section of valuepartnerships.com.
- If you’re interested in participating in PGIP as an individual practitioner, click here to learn more about PGIP physician organizations.
Some pre-COVID-19 utilization management requirements to resume July 1
With the end of the COVID-19 public health emergency, or PHE, Blue Cross Blue Shield of Michigan and Blue Care Network will reinstate certain utilization management requirements that were in effect before the PHE.
Below are the changes and when they’ll go into effect.
|
Topic |
During the PHE |
Change |
For BCN Advantage℠ members, services from providers who aren’t associated with the member’s plan |
Prior authorization requests were approved without clinical review. |
Clinical review will be required for dates of service on or after July 1, 2023. |
For all members, acute medical inpatient admissions related to COVID-19, flu, pneumonia or respiratory syncytial virus |
Prior authorization requests were approved without clinical review. |
Clinical review will be required for admissions on or after July 1, 2023. |
Appeal of prior authorization determinations made by Blue Cross or BCN for any service |
The time frames for submitting appeals were waived. |
The normal time frames for submitting appeals will be reinstituted starting July 1, 2023.
Refer to the denial letters for time frames. |
2 additional facilities join our crisis services program
In case you missed this article in the May-June issue of Hospital and Physician Update, we’re reprinting it here. To subscribe to Hospital and Physician Update, a newsletter designed for participating physicians and hospital executives — or to subscribe to other provider newsletters — click here.
We’re continuing to grow our crisis services program, offering members who are experiencing a mental health crisis a wider array of appropriate options. Since we last wrote about our crisis services in our provider newsletters earlier this year, two additional facilities — Network 180 and Integrated Services of Kalamazoo — have joined the program.
Our research with participating facilities has found that members who visit a crisis services location are directed to outpatient services, rather than an emergency department, most of the time and are receiving behavioral health services more quickly. For example, Common Ground is directing them to an outpatient facility 75% of the time.
“This is not only helping our members receive appropriate care but can help them save time and money,” said William Beecroft, M.D., medical director of behavioral health for Blue Cross Blue Shield of Michigan. “Emergency departments are among the most expensive care settings and an emergency department visit can be very time-consuming.”
See the table below for the current crisis locations, service areas and available care options.
Location |
On-site service area |
Available care options |
Common Ground Resources and Crisis Center
1200 N. Telegraph Road, Building 32E, Pontiac, MI 48341
1-800-231-1127 |
Genesee County, Oakland County, Wayne County |
Psychiatric urgent care
(Virtual only)
Mobile crisis
Crisis stabilization (on-site) |
Hegira Health’s COPE
33505 Schoolcraft Road, Livonia, MI 48150
734-721-0200 |
Wayne County |
Psychiatric urgent care (on-site)
Mobile crisis
Crisis stabilization (on-site)
Crisis residential (on-site) |
Integrated Services of Kalamazoo
615 W. Crosstown Parkway
Kalamazoo, MI 49007
269-373-6000 |
Kalamazoo County |
Psychiatric urgent care
Mobile crisis |
Network 180
790 Fuller Ave., NE
Grand Rapids, MI 49503
616-333-1000 |
Kent County |
Mobile crisis |
New Oakland Family Centers
(Various service locations)
1-800-395-3223 |
Ann Arbor, Center Line, Clarkston, Clinton Township, Farmington Hills, Flint, Grand Rapids, Livonia, Okemos, Pontiac, Port Huron, Southgate, Warren |
Mobile crisis
Crisis residential (on-site) |
Pine Rest
300 68th St. SE, Building E, Entrance E1, Grand Rapids, MI 49548
616-455-9200 |
Kent County, Ottawa County |
Psychiatric urgent care (on-site only) |
If you’re a health care provider interested in joining this program, send an email to Dr. William Beecroft at wbeecroft@bcbsm.com or William Pompos at wpompos@bcbsm.com.
Changes to maternity support program include new name,
menopause support
Last year, Blue Cross Blue Shield of Michigan and Blue Care Network began working with Maven, an independent company, to provide a Family Building and Maternity Support Solution.
We’ve renamed this solution the Family Building and Women’s Health Support Solution and enhanced it, as follows:
Maternity program
Starting in July 2023, members who have coverage through Blue Cross and BCN commercial self-funded groups that purchase this program will receive maternity support during the nine months of pregnancy and for one year postpartum.
The program will still end at three months postpartum for members who have individual coverage or coverage through Blue Cross and BCN commercial fully insured groups.
For additional details about this program, see the October 2022 Record and the November-December 2022 BCN Provider News.
Menopause program
In July, members who are experiencing physical and mental symptoms related to menopause can get access to expert advice and resources. This program will be available to all members who have individual coverage or coverage through Blue Cross and BCN commercial fully insured groups. It’s also available to members who have coverage through self-funded groups that purchase this program.
Menopause program support includes:
- Early identification of menopausal symptoms and treatment guidance
- 24/7 virtual access to a coaching care team that specializes in perimenopause, menopause and postmenopause
- Within the Maven mobile app, guided education and access to communities for connecting with others in the same stage of life
- One-on-one mental health support throughout the menopausal journey
Similar to other Maven solutions, the menopause program will include access to the following:
- A dedicated care advocate who can provide personalized, one-on-one support to answer questions, recommend the right types of care for specific needs, and help members find high-quality, in-network providers
- Personalized resources, including clinically approved articles, community forums to engage with others on similar journeys and classes led by clinical professionals
- Clinical virtual support through 24/7 on-demand video appointments available within one hour. Members can speak with top-rated clinical coaches,** including OB-GYNs, mental health specialists and career coaches. Appointments are available in more than 35 languages. A chat option is also available.
**Maven coaches don’t replace in-person care or relationships with established care teams and providers. They’re additional resources.
Maven is an independent company supporting Blue Cross Blue Shield of Michigan and Blue Care Network by providing family building and women’s health support services.
Bronson Internal Medicine: Embracing team-based care to support patients

Dr. Andrew Chen stands with medical assistants Sara Harmen, left, and Kristin Goff.
This is the third article in a series highlighting some top performers in the Patient-Centered Medical Home Designation Program.
In addressing the needs of a wide variety of patients, Bronson Internal Medicine in Kalamazoo places a high value on the team-based approach — an integral part of the Patient-Centered Medical Home program.
A PCMH practice since 2016, Bronson has nine primary care providers and one nurse practitioner, supported by a social worker, nurse care manager and pharmacist. Having an extended care team is especially helpful when treating patients with chronic conditions or social factors that affect their health.
More recently, the practice also joined the Collaborative Care Designation Program, which builds on the PCMH foundation. The program uses the services of a behavioral health care manager and consulting psychiatrist in addition to a primary care provider, to help ensure that behavioral health needs are met.
“Patients are more complex today, with more challenging needs, and they’re living longer,” said Dr. Andrew Chen, who joined the practice in 2021 after completing his residency. “Team-based care will be a necessity moving forward.”
PCMH successes
Dr. Chen pointed to the following processes as being the most instrumental in the practice’s success:
- Using a team-based care model
- Following evidence-based medicine
- Trusting the team to support patients in managing chronic conditions and behavioral health needs
- Providing same-day access to patients to avoid unnecessary emergency department and urgent care visits
Also, the practice uses the Epic medical records system, which provides real-time reminders of gaps in care and the services patients need. The system also provides the MyChart portal, allowing patients direct communication access to their health care providers.
Telemedicine is another factor that’s been crucial to the success of the practice, particularly during the COVID-19 pandemic. It’s allowed providers to meet with patients virtually for various medical needs, counseling related to medications, lab results and mental health needs, as well as for COVID-19 follow-up.
Bronson Internal Medicine is also part of the Provider-Delivered Care Management program in which care management is delivered in the physician’s office, provided by trained care team members who work in conjunction with the physician. “This has allowed providers to have access to quick communications about the patient’s plan of care and how care management could support them,” Dr. Chen said.
Advice for other practices
Asked if he had any advice for practices working to implement PCMH capabilities, Dr. Chen replied: “It’s important for providers to trust and utilize the ancillary staff such as social workers, complex care managers for chronic conditions, behavioral health care managers for mental health issues and pharmacy staff for medication issues. It’s also important to delegate follow-up when appropriate and refer patients with complex conditions who need support to care management.”
Other articles in the series
Check out the following two articles, which appeared in the April and May issues of The Record.
If you’d like to learn more about becoming a PCMH-designated practice, talk with your physician organization or send an email to valuepartnerships@bcbsm.com.
Collaborative Care: A closer look
A patient and behavioral health care specialist at Bronson Medical Group and a doctor with Bronson Internal Medicine & Pediatrics were recently featured in a Blue Cross Blue Shield of Michigan blog and video that highlights the Collaborative Care Program. Check out Expanding Mental Health Care to More Michiganders on MI Blues Perspectives for more information.
Providers to receive denial for incorrect coding of electroencephalogram and ultrasound screening for abdominal aortic aneurysm, starting in September
What you need to know
Health care providers need to code correctly for electroencephalogram and abdominal aortic aneurysm screening to avoid a claim denial.
Electroencephalogram isn’t covered with a headache-only diagnosis. And the codes below shouldn’t be billed when the only diagnosis code on the claim is for headache or migraine. Beginning in September 2023, you may receive a denial when these codes are billed with a headache-only diagnoses:
- *95812 — Electroencephalogram (EEG) extended monitoring; 41 to 60 minutes
- *95813 — Electroencephalogram (EEG) extended monitoring; 61 to 119 minutes
- *95816 — Electroencephalogram (EEG); including recording awake and drowsy
- *95819 — Electroencephalogram (EEG); including recording awake and asleep
- *95822 — Electroencephalogram (EEG); recording in coma or sleep only
Abdominal aortic aneurysm screening
Blue Cross Blue Shield of Michigan payment policy for abdominal aortic aneurysm screening study aligns with Centers for Medicare & Medicaid Services guidelines. Beginning in September 2023, you may receive a denial when an abdominal aortic aneurysm (CPT *76706) is billed for a male patient 65 years or older, but younger than 76, when a family history of abdominal aortic aneurysm or history of smoking isn’t present on the claim.
Discuss health concerns addressed in Medicare Health Outcomes Survey with your patients
Beginning in July, some of your Blue Cross Blue Shield of Michigan and Blue Care Network patients with Medicare Advantage plans may receive the annual Health Outcomes Survey, or HOS, conducted by Centers for Medicare & Medicaid Services. The survey will ask them about the status of their physical and mental health, and whether they’ve been advised by their physician about fall prevention, managing urinary incontinence and physical activity.
How you can make a difference
The interactions you have with your patients directly affect the responses on the survey. Some patients may need your encouragement to discuss their concerns during their annual physical. Having discussions and advising them on the topics covered in the HOS improves your patients’ quality of life, engagement in their health and experience with your practice.
Here are some suggestions for topics to discuss at annual physicals:
- Review and address any physical or emotional wellness concerns.
- Discuss and advise on appropriate exercise.
- Develop treatment plans with patients to address incontinence.
- Discuss ways to prevent falls and improve balance.
How Blue Cross and BCN are supporting you
- Offering members incentives for annual wellness visits
- Sending members emails and letters to encourage them to have these conversations with you
- Providing health care providers with live and on-demand webinars to address incontinence, physical activity and fall prevention (See the “Find out more about HOS” section below for webinar information.)
- Providing members with the SilverSneakers® program
- Providing informational resources
Find out more about HOS
Our CMS Star measures course has a module with more detail about the Health Outcomes Survey. Click here to log in to the provider training website and search for the course. Use the keyword “HOS.”
If you don’t already have access to the provider training website, you can easily create an account by clicking here. We recommend you use the same email address you use to communicate with Blue Cross Blue Shield of Michigan when creating the account.
You can also check out our Health Outcomes Survey tip sheet for sample survey questions and tips for success.
Blue Cross and BCN to use Audaire Health provider portal to capture clinical outcomes for CAR-T cell therapy drugs
Starting July 1, 2023, Blue Cross Blue Shield of Michigan and Blue Care Network will use the Audaire Health™ provider portal to track and capture clinical outcomes for CAR-T cell therapy drugs for Blue Cross commercial and BCN commercial members.
The data entered in the portal will enable Blue Cross and BCN to capture and assess the clinical benefit of these therapies.
The Audaire portal will replace the manual reporting process for clinical outcomes for CAR-T cell therapy drugs, thereby reducing administrative burden. Blue Cross or BCN will no longer fax forms to prescribing health care providers on an annual basis for them to enter clinical outcome information and send back to us.
Which CAR-T cell therapy drugs are affected?
Starting July 1, Blue Cross and BCN will require providers to enter clinical outcomes into the Audaire provider portal for the following CAR-T cell therapies, which are covered under members’ medical benefits.
|
HCPCS code |
Brand name |
Generic name |
Q2055 |
Abecma® |
idecabtagene vicleucel |
Q2054 |
Breyanzi® |
lisocabtagene maraleucel |
Q2056 |
Carvykti™ |
ciltacabtagene autoleucel |
Q2042 |
Kymriah® |
tisagenlecleucel |
Q2053 |
Tecartus® |
brexucabtagene autoleucel |
Q2041 |
Yescarta® |
axicabtagene ciloleucel |
Notes:
- Current requirements will continue to apply to these drugs. Continue to submit prior authorization requests through the NovoLogix® online tool. (To access NovoLogix, log in to our provider portal, availity.com,** click on Payer Spaces and then click on the BCBSM and BCN logo; this will take you to the Blue Cross and BCN payer space, where you’ll find links to the NovoLogix tools on the Applications tab. If you don’t have access to Availity®, see the Register for web tools page on bcbsm.com.)
- Providers must enter clinical information in the Audaire provider portal for any additional CAR-T cell therapy drugs approved by the Food and Drug Administration after July 1, 2023.
What will change on July 1?
- The first time Blue Cross or BCN approves your prior authorization request for one of these therapies, you (the requesting provider) must attest that you’ll enter clinical outcome information in the Audaire provider portal. Attestation is required for the therapies to be covered by a member’s benefit. (See How should you prepare for this change? below to learn more about attestation.)
- We’ll automatically add basic information to the Audaire provider portal for members approved for one of these therapies.
- We’ll also add basic information for members approved for one of these therapies from Jan. 1, 2022, through June 30, 2023. An Audaire representative will contact and ask requesting providers to enter clinical outcome information in its provider portal for these members.
- Audaire will send emails on a regular basis to remind providers to submit clinical information. Emails will come from hello@audaire.com, and they’ll include a direct link to the portal for easy access.
Providers can use either of these submission methods:
- Enter clinical information in the easy-to-use survey format in the Audaire provider portal.
- Call 512-643-5099. An Audaire representative will enter the clinical information on your behalf.
Note: Call 512‑643‑5099 to schedule an appointment with an Audaire representative to learn how to enter information in the portal.
How should you prepare for this change?
You don’t need to take action.
The first time Blue Cross or BCN approves your prior authorization request for one of these therapies, an Audaire representative will reach out to you to set up a 30-minute phone call during which they’ll:
- Create your Audaire Health profile, which will complete your attestation.
- Provide training on how to use the portal.
- Answer your questions.
An Audaire representative will also reach out to you if you have patients for whom we approved an authorization request for one of these therapies before July 1 and who have active coverage with Blue Cross or BCN.
Why are we making this change?
CAR-T cell therapies are high-cost treatments, and Blue Cross and BCN recognize the value and therapeutic promise these therapies hold. The goal of collecting this data is to ensure member access to therapies while maintaining affordability.
Do other drugs require tracking in the Audaire provider portal?
Blue Cross and BCN also require providers to track clinical outcomes for commercial members for the following high-cost spinal muscular atrophy therapies:
- Zolgensma® (onasemnogene abeparvovec-xioi)
- Spinraza® (nusinersen)
- Evrysdi® (risdiplam)
For additional details, see the October 2022 Record article and the November-December 2022 BCN Provider News article.
Questions?
Email questions to Allison Olmsted, Pharm.D., at aolmsted@bcbsm.com.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Audaire Health is a contracted independent company that provides select services to Blue Cross and BCN commercial members.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services.
Starting July 1, we’ll change how we pay for certain drugs that must be administered by a health care provider
Our goal at Blue Cross Blue Shield of Michigan and Blue Care Network is to provide our members with safe, high-quality prescription drug therapies. We continually review prescription drugs to help ensure we provide the best value for our members, control costs and make sure our members are using the right drug for the right situation. Starting July 1, 2023, we’ll change how we pay for certain drugs.
We’ll no longer pay for the drugs listed below with the member’s prescription drug benefit. Since these drugs should only be administered by a health care provider, we’ll pay for them under the member’s medical benefit. Our prescription drug benefits only pay for drugs that can be self-administered by the patient, per prescription labeling approved by the U.S. Food and Drug Administration.
This change affects all pharmacy drug lists where the drug is currently covered by the pharmacy benefit.
Drugs paid for only by medical benefits, starting July 1, 2023 |
Generic name |
Brand name |
Common use |
Lanreotide |
Lanreotide (brand) |
Acromegaly, carcinoid syndrome, gastroenteropancreatic neuroendocrine tumors |
Somatuline® Depot |
Octreotide |
Sandostatin® LAR® |
Pasireotide |
Signifor® LAR |
Acromegaly, Cushing’s disease |
We’ll send letters to affected groups, members and their providers about this change. And we’ll advise members to talk with their doctor about continuing to receive their drug therapies and how certain drugs should be billed to their medical benefit, starting July 1.
Tzield to require prior authorization for URMBT members with Blue Cross non-Medicare plans
For dates of service on or after July 10, 2023, Tzield™ (teplizumab-mzwv), HCPCS codes J3590/C9149, will require prior authorization through the NovoLogix® online tool for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans.
This drug is covered under the medical benefit.
The prior authorization requirement applies only when Tzield is administered in an outpatient setting.
Note: Prior authorization doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714).
How to submit authorization requests
Submit prior authorization requests through NovoLogix. It offers real-time status checks and immediate approvals for certain medications.
To access NovoLogix, log in to our provider portal (availity.com),** click on Payer Spaces in the menu bar and then click on the BCBSM and BCN logo. You’ll find links to the NovoLogix tools on the Applications tab.
If you need to request access to our provider portal, follow the instructions on the Register for web tools webpage on bcbsm.com/providers.
More about the authorization requirement
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans, see:
We’ll update the pertinent drug lists to reflect the information in this message prior to the effective date.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services.
Questionnaires in e-referral system have been updated
What you need to know
Several questionnaires in the e-referral system have been updated. Providers can preview questionnaires by visiting ereferrals.bcbsm.com.
On April 24, 2023, we updated questionnaires in the e-referral system. We also updated the corresponding preview questionnaires on ereferrals.bcbsm.com.
As a reminder, we use our authorization criteria, our medical policies and your answers to the questionnaires in the e-referral system when making utilization management determinations on your prior authorization requests.
Updated questionnaires
We updated the following questionnaires:
Questionnaire |
Opens for |
Updates |
Cosmetic and reconstructive surgery |
- Medicare Plus Blue℠
- BCN commercial
- BCN Advantage℠
|
- Updated some questions
- Deleted a question
|
Otoplasty |
- BCN commercial
- BCN Advantage
|
Updated a question |
Pediatric feeding program |
BCN commercial |
Updated a question |
Rhinoplasty |
- Medicare Plus Blue
- BCN commercial
- BCN Advantage
|
Deleted a question |
Preview questionnaires
Preview questionnaires show the questions in the e-referral system so you can prepare your answers ahead of time.
To find the preview questionnaires, go to ereferrals.bcbsm.com and:
- For Medicare Plus Blue: Click on Blue Cross and then click on Authorization Requirements & Criteria. Scroll down and look under the Authorization criteria and preview questionnaires – Medicare Plus Blue heading.
- For BCN: Click on BCN and then click on Authorization Requirements & Criteria. Scroll down and look under the Authorization criteria and preview questionnaires heading.
Authorization criteria and medical policies
The pertinent authorization criteria and medical policies are also available on the Authorization Requirements & Criteria page.
Blue Cross covers medically necessary care
As a reminder, Blue Cross Blue Shield of Michigan covers medically necessary care, defined in the Blue Cross Blue Shield of Michigan Commercial Provider Manual as procedures, treatments, supplies, devices, equipment, facilities or drugs (all services) that a medical practitioner, exercising prudent clinical judgment, would provide to a patient for the purpose of preventing, evaluating, diagnosing or treating an illness, injury or disease or its symptoms.
Medically necessary care must also be:
- In accordance with generally accepted standards of medical practice
- Clinically appropriate in terms of type, frequency, extent, site and duration and considered effective for the patient’s illness, injury or disease
- Not primarily for the convenience of the patient, physician or other health care provider
- Not more costly than an alternative service or sequence of services at least as likely to produce equivalent therapeutic or diagnostic results as to the diagnosis or treatment of that patient’s illness, injury or disease
The provider manual further states that these standards are based on credible scientific evidence published in peer-reviewed medical literature generally recognized by the relevant medical community, physician specialty society recommendations, the views of medical practitioners working in relevant clinical areas, and other factors.
Physical medicine services must be:
- Medically necessary and appropriate for the patient’s acute diagnosis due to recent illness, injury or distinct aggravation of a condition or surgery
- Given for a condition that can be significantly improved in a reasonable and generally predictable period of time
- Documented in the patient’s medical record
Providers with questions should call Provider Services at 313-225-9000 or 1-800-344-8525. Patients with questions should contact Customer Service at the number on the back of their member ID cards.
Lunch and learn webinars for physicians and coders focus on risk adjustment, coding
We’re offering live, 30-minute educational webinars that will provide updated information on documentation and coding for common challenging diagnoses. Webinars include an opportunity to ask questions.
Here’s our current schedule and tentative topics for the webinars. Each session starts at noon Eastern time. Log in to the provider training website to register for sessions that work with your schedule.
Session date |
Topic |
June 21 |
Coding diabetes and hypertension |
July 19 |
Coding heart disease and vascular |
Aug. 16 |
Medical record documentation and coding MEAT |
Sept. 20 |
Coding tips for COPD and asthma |
Oct. 18 |
ICD-10-CM updates and changes for 2024 |
Nov. 15 |
Coding chronic kidney disease and rheumatoid arthritis |
Dec. 13 |
CPT coding scenarios for 2024 |
If you haven’t already registered for the provider training website, follow these steps:
- Click here to register.
- Complete the registration. We recommend using the same email you use to communicate with Blue Cross Blue Shield of Michigan for other needs. This will become your login ID.
Locating a session
Click here if you are already registered for the provider training website. On the provider training website, look in the Event Calendar or use the search feature using the keyword “lunch” to quickly locate all 2023 sessions.
See screenshots below for more details.
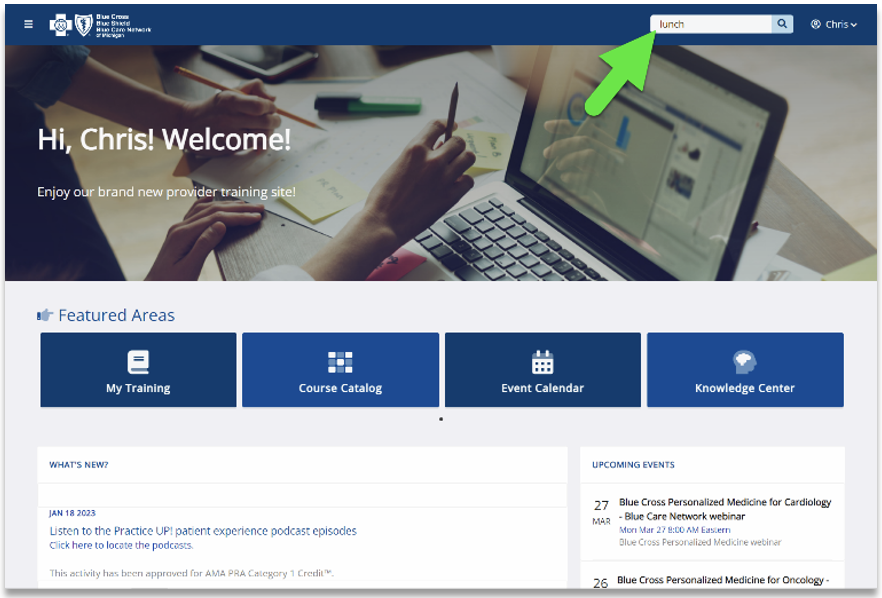
Previous sessions
You can also listen to previously recorded sessions. Check out the following:
Date |
Topic |
April 26 |
HCC and risk adjustment coding scenarios |
May 17 |
Coding neoplasms |
For more information
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding a session or website registration, email ProviderTraining@bcbsm.com.
Reminder: Register for 2023 virtual provider symposium sessions
Action item
Register for one of the upcoming virtual provider symposium sessions listed below.
This year’s virtual provider symposiums started in May and continue in June. Physicians, physician assistants, nurse practitioners, nurses and coders can receive continuing education credits for attending. You’re welcome to register for any session listed below.
To register:
- Click here to log in to the provider training website. If you don’t already have access, click here to register. We recommend you use the same email address you use to communicate with Blue Cross Blue Shield of Michigan for other needs when creating the account.
- Open the Event Calendar to sign up for any of the following upcoming sessions.
Here’s a look at the upcoming sessions, who they’ve been designed for and the dates and times.
Reach for the Stars-HEDIS®/Star Measure Overview: For physicians and office staff responsible for closing gaps in care related to quality adult measures
Session |
Date |
Time |
Reach for the Stars-HEDIS®/Star Measure Overview |
June 1 |
8 to 9:30 a.m. |
Reach for the Stars-HEDIS®/Star Measure Overview |
June 6 |
Noon to 1:30 p.m. |
Patient Experience: For physicians and office staff responsible for creating positive patient experiences. Learn how to ensure your practice has the knowledge and tools needed to set and meet patients’ expectations.
Session |
Date |
Time |
Patient Experience — Best Practices for the New Normal |
June 8 |
9 to 10:30 a.m. |
Coding Complex Cases: For physicians, coders, billers and administrative staff
Session |
Date |
Time |
2023 CPT Coding Updates and Coding Complex Cases |
June 7 |
9 to 10 a.m. |
2023 CPT Coding Updates and Coding Complex Cases |
June 20 |
Noon to 1 p.m. |
Questions?
Contact Ellen Kraft at ekraft@bcbsm.com if you have questions about the sessions. Contact the Provider Training team at ProviderTraining@bcbsm.com if you have questions about registration or using the provider training website.
HEDIS® is a registered trademark of the National Committee for Quality Assurance.
Accreditation Statement: This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the Minnesota Medical Association and Blue Cross Blue Shield of Michigan. The Minnesota Medical Association (MMA) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
CME Statement: The Minnesota Medical Association designates this internet live activity for a maximum of 4 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Resources for cancer screening and prevention available
This is part of an ongoing series of articles focusing on the tools and resources available to help FEP members manage their health.
The Michigan Quality Improvement Consortium provides guidelines for preventive services for adults ages 18 through 49** and 50 and older.** These guidelines include information on screenings for colorectal, cervical and breast cancers.
Here are some resources from the American Cancer Society, Centers for Disease Control and Prevention, Department of Health and Human Services, and American Lung Association:
Blue Cross and Blue Shield Federal Employee Program® benefits have no out-of-pocket cost for colorectal, cervical and breast cancer screenings when done by a Preferred provider.
FEP members also have benefits for smoking and tobacco cessation treatment.
Providers and members can call Customer Service at 1-800-482-3600 or go online to fepblue.org if they have questions about FEP benefits.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.

Some pre-COVID-19 utilization management requirements to resume July 1
With the end of the COVID-19 public health emergency, or PHE, Blue Cross Blue Shield of Michigan and Blue Care Network will reinstate certain utilization management requirements that were in effect before the PHE.
Below are the changes and when they’ll go into effect.
|
Topic |
During the PHE |
Change |
For BCN Advantage℠ members, services from providers who aren’t associated with the member’s plan |
Prior authorization requests were approved without clinical review. |
Clinical review will be required for dates of service on or after July 1, 2023. |
For all members, acute medical inpatient admissions related to COVID-19, flu, pneumonia or respiratory syncytial virus |
Prior authorization requests were approved without clinical review. |
Clinical review will be required for admissions on or after July 1, 2023. |
Appeal of prior authorization determinations made by Blue Cross or BCN for any service |
The time frames for submitting appeals were waived. |
The normal time frames for submitting appeals will be reinstituted starting July 1, 2023.
Refer to the denial letters for time frames. |
2 additional facilities join our crisis services program
In case you missed this article in the May-June issue of Hospital and Physician Update, we’re reprinting it here. To subscribe to Hospital and Physician Update, a newsletter designed for participating physicians and hospital executives — or to subscribe to other provider newsletters — click here.
We’re continuing to grow our crisis services program, offering members who are experiencing a mental health crisis a wider array of appropriate options. Since we last wrote about our crisis services in our provider newsletters earlier this year, two additional facilities — Network 180 and Integrated Services of Kalamazoo — have joined the program.
Our research with participating facilities has found that members who visit a crisis services location are directed to outpatient services, rather than an emergency department, most of the time and are receiving behavioral health services more quickly. For example, Common Ground is directing them to an outpatient facility 75% of the time.
“This is not only helping our members receive appropriate care but can help them save time and money,” said William Beecroft, M.D., medical director of behavioral health for Blue Cross Blue Shield of Michigan. “Emergency departments are among the most expensive care settings and an emergency department visit can be very time-consuming.”
See the table below for the current crisis locations, service areas and available care options.
Location |
On-site service area |
Available care options |
Common Ground Resources and Crisis Center
1200 N. Telegraph Road, Building 32E, Pontiac, MI 48341
1-800-231-1127 |
Genesee County, Oakland County, Wayne County |
Psychiatric urgent care
(Virtual only)
Mobile crisis
Crisis stabilization (on-site) |
Hegira Health’s COPE
33505 Schoolcraft Road, Livonia, MI 48150
734-721-0200 |
Wayne County |
Psychiatric urgent care (on-site)
Mobile crisis
Crisis stabilization (on-site)
Crisis residential (on-site) |
Integrated Services of Kalamazoo
615 W. Crosstown Parkway
Kalamazoo, MI 49007
269-373-6000 |
Kalamazoo County |
Psychiatric urgent care
Mobile crisis |
Network 180
790 Fuller Ave., NE
Grand Rapids, MI 49503
616-333-1000 |
Kent County |
Mobile crisis |
New Oakland Family Centers
(Various service locations)
1-800-395-3223 |
Ann Arbor, Center Line, Clarkston, Clinton Township, Farmington Hills, Flint, Grand Rapids, Livonia, Okemos, Pontiac, Port Huron, Southgate, Warren |
Mobile crisis
Crisis residential (on-site) |
Pine Rest
300 68th St. SE, Building E, Entrance E1, Grand Rapids, MI 49548
616-455-9200 |
Kent County, Ottawa County |
Psychiatric urgent care (on-site only) |
If you’re a health care provider interested in joining this program, send an email to Dr. William Beecroft at wbeecroft@bcbsm.com or William Pompos at wpompos@bcbsm.com.
Providers to receive denial for incorrect coding of electroencephalogram and ultrasound screening for abdominal aortic aneurysm, starting in September
What you need to know
Health care providers need to code correctly for electroencephalogram and abdominal aortic aneurysm screening to avoid a claim denial.
Electroencephalogram isn’t covered with a headache-only diagnosis. And the codes below shouldn’t be billed when the only diagnosis code on the claim is for headache or migraine. Beginning in September 2023, you may receive a denial when these codes are billed with a headache-only diagnoses:
- *95812 — Electroencephalogram (EEG) extended monitoring; 41 to 60 minutes
- *95813 — Electroencephalogram (EEG) extended monitoring; 61 to 119 minutes
- *95816 — Electroencephalogram (EEG); including recording awake and drowsy
- *95819 — Electroencephalogram (EEG); including recording awake and asleep
- *95822 — Electroencephalogram (EEG); recording in coma or sleep only
Abdominal aortic aneurysm screening
Blue Cross Blue Shield of Michigan payment policy for abdominal aortic aneurysm screening study aligns with Centers for Medicare & Medicaid Services guidelines. Beginning in September 2023, you may receive a denial when an abdominal aortic aneurysm (CPT *76706) is billed for a male patient 65 years or older, but younger than 76, when a family history of abdominal aortic aneurysm or history of smoking isn’t present on the claim.
Starting July 1, we’ll change how we pay for certain drugs that must be administered by a health care provider
Our goal at Blue Cross Blue Shield of Michigan and Blue Care Network is to provide our members with safe, high-quality prescription drug therapies. We continually review prescription drugs to help ensure we provide the best value for our members, control costs and make sure our members are using the right drug for the right situation. Starting July 1, 2023, we’ll change how we pay for certain drugs.
We’ll no longer pay for the drugs listed below with the member’s prescription drug benefit. Since these drugs should only be administered by a health care provider, we’ll pay for them under the member’s medical benefit. Our prescription drug benefits only pay for drugs that can be self-administered by the patient, per prescription labeling approved by the U.S. Food and Drug Administration.
This change affects all pharmacy drug lists where the drug is currently covered by the pharmacy benefit.
Drugs paid for only by medical benefits, starting July 1, 2023 |
Generic name |
Brand name |
Common use |
Lanreotide |
Lanreotide (brand) |
Acromegaly, carcinoid syndrome, gastroenteropancreatic neuroendocrine tumors |
Somatuline® Depot |
Octreotide |
Sandostatin® LAR® |
Pasireotide |
Signifor® LAR |
Acromegaly, Cushing’s disease |
We’ll send letters to affected groups, members and their providers about this change. And we’ll advise members to talk with their doctor about continuing to receive their drug therapies and how certain drugs should be billed to their medical benefit, starting July 1.
Tzield to require prior authorization for URMBT members with Blue Cross non-Medicare plans
For dates of service on or after July 10, 2023, Tzield™ (teplizumab-mzwv), HCPCS codes J3590/C9149, will require prior authorization through the NovoLogix® online tool for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans.
This drug is covered under the medical benefit.
The prior authorization requirement applies only when Tzield is administered in an outpatient setting.
Note: Prior authorization doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714).
How to submit authorization requests
Submit prior authorization requests through NovoLogix. It offers real-time status checks and immediate approvals for certain medications.
To access NovoLogix, log in to our provider portal (availity.com),** click on Payer Spaces in the menu bar and then click on the BCBSM and BCN logo. You’ll find links to the NovoLogix tools on the Applications tab.
If you need to request access to our provider portal, follow the instructions on the Register for web tools webpage on bcbsm.com/providers.
More about the authorization requirement
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans, see:
We’ll update the pertinent drug lists to reflect the information in this message prior to the effective date.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services.
Blue Cross covers medically necessary care
As a reminder, Blue Cross Blue Shield of Michigan covers medically necessary care, defined in the Blue Cross Blue Shield of Michigan Commercial Provider Manual as procedures, treatments, supplies, devices, equipment, facilities or drugs (all services) that a medical practitioner, exercising prudent clinical judgment, would provide to a patient for the purpose of preventing, evaluating, diagnosing or treating an illness, injury or disease or its symptoms.
Medically necessary care must also be:
- In accordance with generally accepted standards of medical practice
- Clinically appropriate in terms of type, frequency, extent, site and duration and considered effective for the patient’s illness, injury or disease
- Not primarily for the convenience of the patient, physician or other health care provider
- Not more costly than an alternative service or sequence of services at least as likely to produce equivalent therapeutic or diagnostic results as to the diagnosis or treatment of that patient’s illness, injury or disease
The provider manual further states that these standards are based on credible scientific evidence published in peer-reviewed medical literature generally recognized by the relevant medical community, physician specialty society recommendations, the views of medical practitioners working in relevant clinical areas, and other factors.
Physical medicine services must be:
- Medically necessary and appropriate for the patient’s acute diagnosis due to recent illness, injury or distinct aggravation of a condition or surgery
- Given for a condition that can be significantly improved in a reasonable and generally predictable period of time
- Documented in the patient’s medical record
Providers with questions should call Provider Services at 313-225-9000 or 1-800-344-8525. Patients with questions should contact Customer Service at the number on the back of their member ID cards.
Lunch and learn webinars for physicians and coders focus on risk adjustment, coding
We’re offering live, 30-minute educational webinars that will provide updated information on documentation and coding for common challenging diagnoses. Webinars include an opportunity to ask questions.
Here’s our current schedule and tentative topics for the webinars. Each session starts at noon Eastern time. Log in to the provider training website to register for sessions that work with your schedule.
Session date |
Topic |
June 21 |
Coding diabetes and hypertension |
July 19 |
Coding heart disease and vascular |
Aug. 16 |
Medical record documentation and coding MEAT |
Sept. 20 |
Coding tips for COPD and asthma |
Oct. 18 |
ICD-10-CM updates and changes for 2024 |
Nov. 15 |
Coding chronic kidney disease and rheumatoid arthritis |
Dec. 13 |
CPT coding scenarios for 2024 |
If you haven’t already registered for the provider training website, follow these steps:
- Click here to register.
- Complete the registration. We recommend using the same email you use to communicate with Blue Cross Blue Shield of Michigan for other needs. This will become your login ID.
Locating a session
Click here if you are already registered for the provider training website. On the provider training website, look in the Event Calendar or use the search feature using the keyword “lunch” to quickly locate all 2023 sessions.
See screenshots below for more details.


Previous sessions
You can also listen to previously recorded sessions. Check out the following:
Date |
Topic |
April 26 |
HCC and risk adjustment coding scenarios |
May 17 |
Coding neoplasms |
For more information
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding a session or website registration, email ProviderTraining@bcbsm.com.
|



