|
April 2024

Attribution will be expanded to advanced practice providers in April
Starting in April, physician organizations and Blueprint risk-bearing contracted entities will be able to align advanced practice health care providers (certified nurse practitioners and physician assistants) to their provider entities through the Provider Enrollment and Change Self-Service tool in our provider portal (availity.com).**
To access the Provider Enrollment and Change Self-Service tool in our provider portal:
- Log in to availity.com.**
- Under Payer Spaces, click on the BCBSM and BCN logo.
- Click on the Applications tab.
- Scroll down and then click on the tile for Provider Enrollment and Change Self-Service.
Note: Users need to be registered in one of the following roles to access the tool:
- Provider data management
- Provider enrollment and contracting
Advanced practice providers can identify as primary care providers
Advanced practice providers will also be able to identify as primary care providers for our Blue Cross Blue Shield of Michigan commercial PPO and Medicare Plus Blue℠ PPO products during enrollment, which will allow them to be included in Blue Cross’ primary care attribution process used by our Physician Group Incentive Program, Blueprint and other provider value-based programs. Details on advanced practice provider opportunities in our provider value-based programs will be announced at a later date.
When eligible certified nurse practitioners or physician assistants choose to enroll as primary care providers, this will enable Blue Cross members to search for them as primary care providers on the Find a Doctor directory on bcbsm.com. Eligible CNP primary care provider specialties for Blue Cross PPO and Medicare Plus Blue PPO include:
- Acute care nurse practitioner
- Adult care nurse practitioner
- Certified nurse practitioner
- Family nurse practitioner
- Adult care nurse practitioner
- Pediatric nurse practitioner
- Gerontology
Physician assistants will be considered primary care providers based on the specialty of their supervising physicians (also known as the participating physicians) during enrollment. The participating physicians must be M.D.s or D.O.s, actively enrolled with Blue Cross, and fully credentialed and in good standing with the company. Non-Blue Cross M.D.s and D.O.s, or those with an active disciplinary status, can’t be the participating physicians for physician assistants. Eligible physician subspecialties include:
- Adolescent medicine
- Family practice (medicine)
- Family medicine and osteopathic manipulative therapy, or OMT
- General practice
- Geriatric practice
- Internal medicine
- Pediatrics
- Preventive medicine
- Public/health/general
Participating physician data maintenance (for example, changing participating physician) will be managed through the same process used for all other practitioner demographic changes. Physician assistant primary care provider eligibility will be limited to Blue Cross PPO and Medicare Plus Blue PPO at this time. PAs will be eligible to be a Blue Care Network primary care provider at a future date to be determined.
CNPs and PAs identified as primary care providers will be included in our primary care member attribution process for Blue Cross PPO and Medicare Plus Blue PPO. M.D.s and D.O.s will take precedence over the CNPs and PAs in the attribution algorithm. A CNP or PA will never reduce M.D. or D.O. attribution. CNPs and PAs will only receive attributed members when those members haven’t seen an M.D. or D.O. primary care provider for at least 24 months.
Contact Provider Inquiry if you have any questions related to advanced practice providers’ primary care provider eligibility or member attribution implications.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
Reminder: How to check the status of prior authorization requests to share with your patients
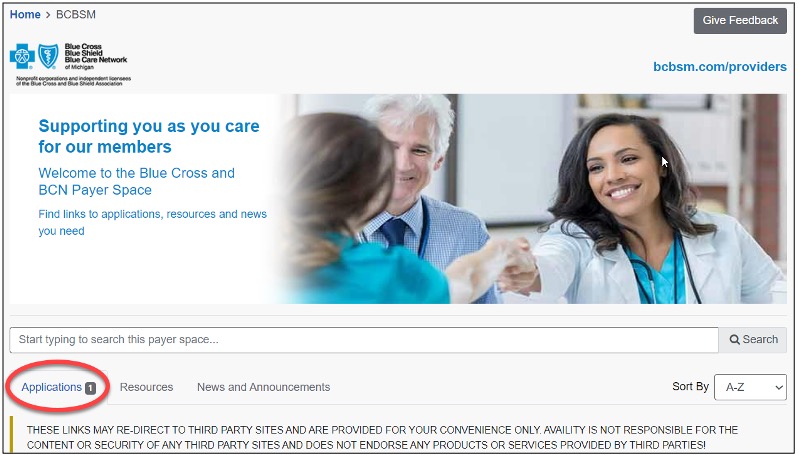
As a reminder, if a patient who has coverage through Blue Cross Blue Shield of Michigan or Blue Care Network asks about the status of a prior authorization request, you can check it by following these steps:
- Log in to our provider portal at availity.com.**
- Click on Payer Spaces in the menu bar, and then click on the BCBSM and BCN logo.
- Click on the applicable tile in the Applications tab through which you submitted the authorization request.
Additional information available for providers
Providers can also find a summary of services that require prior authorization through our Summary of utilization management programs for Michigan providers document on ereferrals.bcbsm.com.
Note: For help using the e-referral tool, go to ereferrals.bcbsm.com and, under Access & Training, click on Training Tools.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
Starting June 1, Blue Cross will no longer cover Sajazir injection
Starting June 1, 2024, Blue Cross Blue Shield of Michigan and Blue Care Network will no longer cover Sajazir™ injection as a pharmacy benefit. Instead, we’ll cover generic icatibant acetate subcutaneous injection. Sajazir is a medication commonly used to treat acute attacks of hereditary angioedema.
Both Sajazir and icatibant acetate subcutaneous injection are generic icatibant acetate products for brand name Firazyr® and are FDA-approved. However, Sajazir is more expensive than other available generic products. It also requires limited distribution through LeMed Specialty Pharmacy©, a nonpreferred specialty pharmacy, whereas the other generic products are available through specialty pharmacies. Our preferred specialty pharmacy is AllianceRx Walgreens Pharmacy.
If your patient requires treatment with Sajazir rather than another generic product after June 1, a medical necessity review will be required.
We’ll notify affected members of these changes and encourage them to talk with you to address any concerns and get a new prescription, if needed.
If you have questions, call the Pharmacy Services Clinical Help Desk at 1-800-437-3803.
We added new on-demand training to provider training site
Action item
Visit our provider training site to find new and updated resources on topics that are important to your role.
Provider Experience continues to offer training resources for health care providers and staff. Our on-demand courses are designed to help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network. We recently started publishing short courses that providers and staff can complete in just a few minutes. Find a quick introduction to our mini module concept.
We also added the following learning opportunity:
- Submitting appeals and peer-to-peer review requests in e-referral: The recording for the March 13, 2024, webinar for inpatient hospital providers is now available on the provider training website. You can also take the e-learning course that includes a simulation of the steps you take to submit requests. Search “e-referral” to find these courses along with others about the e-referral tool.
The goal of our provider training site is to enhance the training experience for providers and staff. Check out the dashboard regularly for announcements as we add more courses, including those with CME offerings.
To access the training site, follow these steps:
- Log in to the provider portal at availity.com.**
- Click on Payer Spaces on the menu bar and then click on the BCBSM and BCN logo.
- Under Applications, click on the Provider Training Site tile.
- Click on Submit on the Select an Organization page.
- Existing users who used the same email address as their provider portal profile email will be directed to the training site. If you used a different email address, contact ProviderTraining@bcbsm.com to update your profile.
If you’re a new training site user, complete the one-time registration by entering your role and creating a password. This allows you to access the training site outside of the provider portal if needed.
If you need assistance navigating the provider training site, email ProviderTraining@bcbsm.com.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
2024 CPT 2nd-quarter update: New codes
The American Medical Association has added 34 new Category III codes and two vaccine codes as part of its CPT second-quarter update. The codes, effective dates and Blue Cross Blue Shield of Michigan’s coverage decisions are below.
Category III
Anesthesia Delivery
Code* |
Change |
Coverage comments |
Effective date |
0887T |
Added |
Not covered |
July 1, 2024 |
Category III
Surgery
Code* |
Change |
Coverage comments |
Effective date |
0867T |
Added |
Not covered |
July 1, 2024 |
0869T |
Added |
Not covered |
July 1, 2024 |
0870T |
Added |
Not covered |
July 1, 2024 |
0871T |
Added |
Not covered |
July 1, 2024 |
0872T |
Added |
Not covered |
July 1, 2024 |
0873T |
Added |
Not covered |
July 1, 2024 |
0874T |
Added |
Not covered |
July 1, 2024 |
0875T |
Added |
Not covered |
July 1, 2024 |
0884T |
Added |
Not covered |
July 1, 2024 |
0885T |
Added |
Not covered |
July 1, 2024 |
0886T |
Added |
Not covered |
July 1, 2024 |
0894T |
Added |
Not covered |
July 1, 2024 |
0895T |
Added |
Not covered |
July 1, 2024 |
0896T |
Added |
Not covered |
July 1, 2024 |
Category III
Medicine
Code* |
Change |
Coverage comments |
Effective date |
0868T |
Added |
Not covered |
July 1, 2024 |
0881T |
Added |
Not covered |
July 1, 2024 |
0882T |
Added |
Not covered |
July 1, 2024 |
0883T |
Added |
Not covered |
July 1, 2024 |
0893T |
Added |
Not covered |
July 1, 2024 |
0897T |
Added |
Not covered |
July 1, 2024 |
Category III
Medicine/Behavioral Health
Code* |
Change |
Coverage comments |
Effective date |
0889T |
Added |
Not covered |
July 1, 2024 |
0890T |
Added |
Not covered |
July 1, 2024 |
0891T |
Added |
Not covered |
July 1, 2024 |
0892T |
Added |
Not covered |
July 1, 2024 |
Category III
Radiology
Code* |
Change |
Coverage comments |
Effective date |
0876T |
Added |
Not covered |
July 1, 2024 |
0877T |
Added |
Not covered |
July 1, 2024 |
0878T |
Added |
Not covered |
July 1, 2024 |
0879T |
Added |
Not covered |
July 1, 2024 |
0880T |
Added |
Not covered |
July 1, 2024 |
0888T |
Added |
Not covered |
July 1, 2024 |
Category III
Radiology/Diagnostic Radiology
Code* |
Change |
Coverage comments |
Effective date |
0898T |
Added |
Not covered |
July 1, 2024 |
0899T |
Added |
Not covered |
July 1, 2024 |
0900T |
Added |
Not covered |
July 1, 2024 |
Medicine Vaccines/Toxoids
|
Code* |
Change |
Coverage comments |
Effective date |
90637 |
Added |
Not covered |
July 1, 2024 |
90638 |
Added |
Not covered |
July 1, 2024 |
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation is done in accordance with all applicable state and federal laws and regulations.
Billing chart: Blue Cross highlights medical, benefit policy changes
You’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart.
This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures.
We'll publish information about new Blue Cross groups or changes to group benefits under the Group Benefit Changes heading.
For more detailed descriptions of the Blue Cross' policies for these procedures, check under the Commercial Policy tab in Benefit Explainer on Availity®. To access this online information:
1. Log in to availity.com.
2 .Click on Payer Spaces on the Availity menu bar.
3. Click on the BCBSM and BCN logo.
4. Click on Benefit Explainer on the Applications tab.
5. Click on the Commercial Policy tab.
6. Click on Topic.
7. Under Topic Criteria, click on the circle for Unique Identifier and click the drop-down arrow next to Choose Identifier Type, then click on HCPCS Code.
8. Enter the procedure code.
9. Click on Finish.
10. Click on Search.
| Code* |
BCBSM changes to:
Basic Benefit and Medical Policy, Group
Variations Payment Policy, Guidelines
|
| POLICY CLARIFICATIONS |
Revenue code 0829 |
Basic benefit and medical policy
Revenue code 0829 is an outpatient dialysis code and will be denied as provider liable when reported on an inpatient claim. Make sure to reference a coding authority, such as the Centers for Medicare & Medicaid Services or the National Uniform Billing Committee, for the appropriate inpatient renal dialysis revenue code range.
A list of acceptable outpatient dialysis revenue codes and condition codes can be found in the “Dialysis” chapter of the provider manual. Please reference our provider portal, Availity® Essentials, for a complete list of acceptable codes when reporting outpatient dialysis services.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services. |
61885, 61886, 61888 |
Basic benefit and medical policy
Procedure codes *61885, *61886 and *61888 are payable in the inpatient, outpatient and ambulatory surgery center locations only. |
69705, 69706
Experimental
0583T |
Basic benefit and medical policy
Balloon dilation of the eustachian tube
The safety and effectiveness of U.S. Food and Drug Administration-approved balloon dilation devices have been established. They may be considered a useful therapeutic option in the treatment of chronic obstructive eustachian tube dysfunction when criteria are met.
The medical policy statement, and inclusionary and exclusionary criteria have been updated, effective March 1, 2024.
Inclusionary and exclusionary guidelines
Inclusions:
Balloon dilation of the eustachian tube for treatment of chronic obstructive eustachian tube dysfunction may be considered established under all the following conditions:
- Adults (age 18 years and older) with symptoms of obstructive eustachian tube dysfunction (aural fullness, aural pressure, otalgia or hearing loss) for three months or longer in one or both ears that significantly affects quality of life or functional health status. Aural fullness and pressure must be present.
- The individual has undergone a comprehensive diagnostic assessment, including patient-reported questionnaires, history and physical exam, tympanometry if the tympanic membrane is intact, nasal endoscopy and comprehensive audiometry, with the following findings:
- Abnormal tympanogram (Type B or C)
- Abnormal tympanic membrane (retracted membrane, effusion, perforation or any other abnormality identified on exam)
- Failure to respond to appropriate medical management of potential co-occurring conditions, if any, such as allergic rhinitis, rhinosinusitis and laryngopharyngeal reflux, including four to six weeks of a nasal steroid spray, if indicated.
- Other causes of aural fullness such as temporomandibular joint disorders, extrinsic obstruction of the eustachian tube, superior semicircular canal dehiscence and endolymphatic hydrops have been ruled out.
- If the individual had a history of tympanostomy tube placement, symptoms of obstructive eustachian tube dysfunction should have improved while tubes were patent.
- The individual doesn’t have patulous eustachian tube dysfunction or another contraindication to the procedure. (See Policy Guidelines.)
- The individual’s eustachian tube dysfunction has been shown to be reversible. (See Policy Guidelines.)
- Symptoms are continuous rather than episodic (e.g., symptoms occur only in response to barochallenge, such as pressure changes while flying).
- The individual hasn’t had a previous BDET procedure.
- In certain situations, consideration may be given to individuals younger than 18 years of age. The most likely scenario is older children or adolescents who have failed standard treatment with grommet (ventilation or tympanostomy tube insertion), adenoidectomy or both.
Reversibility of eustachian tube dysfunction:
Reversibility of eustachian tube dysfunction can be demonstrated by several means, including any of the following:
- The individual states that they are able to relieve the pressure by performing a Valsalva maneuver to “pop” their ears.
- Performing a Valsalva maneuver produces temporary improvement of the individual’s tympanogram to Type A tympanogram.
- Performing a Valsalva maneuver causes the member’s middle ear to aerate, which is indicated by the provider visualizing lateral movement of the tympanic membrane on otoscopy.
Balloon dilation of the eustachian tube used in combination with other procedures:
- Individuals undergoing BDET concurrent with sinus ostial dilation should meet the same diagnostic criteria for BDET as those undergoing BDET alone.
- Individuals with a middle ear effusion at the time of BDET may benefit from concurrent myringotomy with or without tympanostomy tube placement.
Exclusions:
Balloon dilation of the eustachian tube is considered experimental if the above criteria aren’t met.
Symptoms of obstructive eustachian tube dysfunction may include aural fullness, aural pressure, otalgia and hearing loss. Nearly all individuals will have aural fullness and aural pressure. Many individuals will have otalgia, but hearing loss may not be present in all individuals (e.g., patients with Type C tympanograms).
Contraindications:
The following individuals should not be considered for balloon dilation of the eustachian tube:
- Individuals with patulous eustachian tube dysfunction
- A diagnosis of patulous ETD is suggested by symptoms of autophony of voice, audible respirations, pulsatile tinnitus or aural fullness.
- Individuals with extrinsic reversible or irreversible causes of eustachian tube dysfunction, including, but not limited to:
- Craniofacial syndromes, including cleft palate spectrum
- Neoplasms causing extrinsic obstruction of the eustachian tube
- History of radiation therapy to the nasopharynx
- Enlarged adenoid pads
- Nasopharyngeal mass
- Neuromuscular disorders that lead to hypotonia or ineffective eustachian tube dynamic opening
- Systemic mucosal or autoimmune inflammatory disease affecting the mucosa of the nasopharynx and eustachian tube (e.g. Samter’s triad, Wegener’s disease, mucosal pemphigus) that is ongoing or active (i.e., not in remission)
- Individuals with aural fullness but normal exam and tympanogram
- Individuals with chronic and severe atelectatic ears
|
81162, 81163, 81164, 81165, 81166, 81167, 81212, 81215, 81216, 81217, 81301, 81408, 81432, 81479,** 0037U, 0172U, 0239U
Experimental
0129U
**Unlisted code |
Basic benefit and medical policy
Germline and somatic biomarker testing for targeted treatment in ovarian cancer
The clinical utility of germline BRCA1/2 variant analysis is considered established for individuals with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer to select treatment with FDA-approved targeted therapies.
The clinical utility of somatic BRCA1/2 variant analysis using tumor tissue is considered established for individuals with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer to select treatment with FDA-approved targeted therapies.
All other uses of germline and somatic BRCA1/2 variant analysis to guide targeted therapy for ovarian, fallopian tube or primary peritoneal cancer are considered experimental.
Homologous recombination deficiency, or HRD, of tumor tissue for analysis of tumor tissue or circulating tumor DNA testing (liquid biopsy) is considered established for individuals with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer to select treatment with FDA-approved targeted therapies.
All other uses of HRD testing of tumor tissue to guide targeted therapy for ovarian, fallopian tube or primary peritoneal cancer are considered experimental.
BRCA1/2 variant analysis using circulating tumor DNA (liquid biopsy) is considered established for individuals with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer to select treatment with FDA-approved targeted therapies when tissue-based analysis isn’t clinically feasible.
All other uses of circulating tumor DNA testing (liquid biopsy) to guide targeted therapy in individuals with ovarian, fallopian tube or primary peritoneal cancer are considered experimental.
Simultaneous testing using liquid and tumor biopsies (outside of paired or concurrent somatic-germline testing) to guide treatment in individuals with ovarian, fallopian tube or primary peritoneal cancer is considered experimental, effective March 1, 2024.
Inclusionary and exclusionary guidelines
Inclusions:
The clinical utility of germline and somatic biomarker testing (including liquid biopsy) for targeted treatment in ovarian cancer (BRCA1, BRCA2, homologous recombination deficiency) have been established when any of the following criteria are met:
- Germline BRCA1/2 variant analysis for individuals with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer to select treatment with FDA-approved targeted therapies.
- Somatic BRCA1/2 variant analysis using tumor tissue for individuals with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer to select treatment with FDA-approved targeted therapies.
- Homologous recombination deficiency, or HRD, analysis of tumor tissue or circulating tumor DNA testing (liquid biopsy) is considered established for individuals with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer to select treatment with FDA-approved targeted therapies.
- BRCA1/2 variant analysis using circulating tumor DNA (liquid biopsy) is considered established for individuals with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer to select treatment with FDA-approved targeted therapies when tissue-based analysis isn’t clinically feasible.
Exclusions:
- All other uses of germline and somatic BRCA1/2 variant analysis to guide targeted therapy for ovarian, fallopian tube or primary peritoneal cancer are considered experimental.
- All other uses of HRD testing of tumor tissue to guide targeted therapy for ovarian, fallopian tube or primary peritoneal cancer are considered experimental.
- All other uses of circulating tumor DNA testing (liquid biopsy) to guide targeted therapy in individuals with ovarian, fallopian tube or primary peritoneal cancer are considered experimental.
- Simultaneous testing using liquid and tumor biopsies (outside of paired or concurrent somatic-germline testing) to guide treatment in individuals with ovarian, fallopian tube or primary peritoneal cancer is considered experimental.
|
81191, 81192, 81193, 81194, 81210, 81301, 81445, 81449, 81450, 81451, 81455, 81456, 81479,** 0022U, 0244U, 0250U, 0334U, 0379U, 0037U, 0172U
**Unlisted code |
Basic benefit and medical policy
GT – NGS of multiple genes (panel)
The policy has been updated to cover procedure codes *81449, *81451, *81456, *0244U, *0250U, *0334U and *0379U when criteria are met, effective Jan. 1, 2024.
Next-generation sequence testing of clinically actionable genes, through a multiple-gene panel, may be considered established for solid cancers or hematolymphoid cancers for diagnostic and prognostic purposes, and in guiding the selection of appropriate therapeutic options, when criteria are met.
Next-generation sequencing, or NGS, of clinically actionable genes, through a multiple-gene panel may be considered established for metastatic or advanced cancers when criteria are met.
Inclusionary and exclusionary guidelines
Inclusions:
If there is a medical policy specific to the cancer (type or treatment) and to the appropriate genetic testing, that policy direction supersedes this policy.
Hematolymphoid cancer:
Next-generation sequencing, with a multiple-gene panel test (e.g., CPT codes *81450, *81451, *81455 or *81456), may be considered established when used for diagnostic and prognostic purposes or for guidance in the selection of appropriate targeted FDA therapeutic options for the following conditions.
- Suspected hematolymphoid neoplasms supported by clinical records that reflect an inconclusive diagnosis despite the clinical history, physical examination findings, blood work (e.g., CBC with peripheral smear, chromosome analysis)
- Acute lymphoblastic leukemia
- Acute myelogenous leukemia
- Basophilia
- B-acute lymphocytic leukemia
- B-cell non-Hodgkin lymphoma
- Chronic lymphocytic leukemia
- Chronic myeloid leukemia
- Chronic myeloid proliferative disease
- Essential thrombocythemia or thrombocytosis
- Myelodysplastic syndrome
- Pancytopenia
- Plasma cell dyscrasia
- Pediatric hematologic malignancies
- Polycythemia vera
- Primary myelofibrosis, or PMF, Pre-PMF, or suspicion for PMF
- T-acute lymphocytic leukemia
- T-cell lymphoma, peripheral
Solid cancers:
Next-generation sequencing, or NGS, with a multiple-gene panel test (e.g., CPT codes *81445, *81449, *81455 and *81456), may be considered established when used for diagnostic and prognostic purposes or for guidance in the selection of appropriate targeted FDA-therapeutic options for any of the following conditions:
- Metastatic cancers
- Inoperable locally advanced cancers
- Refractory cancers
- Recurrent cancers
- When diagnosis can’t be made by histopathologic means alone (e.g., sarcomas, neurologic neoplasms, etc.)
- NGS testing for adjuvant therapy in non-advanced cancers may be considered, if consistent with FDA-approved indications
Tumor agnostic therapy:
Next-generation sequencing, or NGS, with a multiple-gene panel test may be considered established when the following criteria are met:
Adult and pediatric individuals for whom there are no satisfactory options in the treatment of metastatic or unresectable solid tumors, or disease progression following prior treatment.
Note: Tumor agnostic therapy is established for the following genetic variants:
- BRAF V600E or V600K variants
- Mismatch repair deficient, or dMMR, or microsatellite instability-high, or MSI-H
- Tumor mutational burden-high, or TMB-H, (≥10 mutations/mega base [mut/Mb])
- Neurotrophic tyrosine receptor kinase, or NTRK 1/2/3, gene fusion
- Programmed cell death ligand 1, or PD-L1
Proprietary laboratory analyses, or PLA, testing:
A PLA test is considered established when both the following criteria are met:
- Biomarker confirmation is required by an FDA-approved or cleared test before initiating treatment (as described in the FDA prescribing label of the therapeutic in the section “Indications and Usage”).
- The test is an FDA-approved companion diagnostic.
Information regarding FDA-approved companion diagnostic tests should be obtained from the FDA “List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools)” website: fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools**
For accuracy, access the information directly from the FDA site because the website is updated frequently.
Exclusions:
- Next-generation sequencing, with multiple-gene panel testing (five to 50 genes; or 51 or more genes) when the above criteria aren’t met
- Concurrent ordering of more than one multiple-gene panel test
**Blue Cross Blue Shield of Michigan doesn’t own or control this website. |
A2021
Additional covered codes:
15271-15278, 15777, A2001, A2002, A2004-A2018, A4100, A6010, A6011, A6021-A6023, Q4100-Q4108, Q4110, Q4113, Q4114, Q4116-Q4118, Q4121, Q4122, Q4124, Q4127, Q4128, Q4130, Q4135, Q4136, Q4147, Q4149, Q4158, Q4161, Q4164-Q4166, Q4182, Q4195, Q4196, Q4203
Experimental codes:
A2019, A2020, C1832, Q4111, Q4112, Q4115, Q4123, Q4125, Q4126, Q4134, Q4141-Q4143, Q4146, Q4152, Q4167, Q4175-Q4180, Q4193, Q4197,Q4200, Q4202, Q4220, Q4222, Q4226, Q4238 |
Basic benefit and medical policy
Skin and tissue substitutes
The policy has been updated to cover procedure code A2021 when criteria are met, effective Nov. 1, 2023.
The safety and effectiveness of skin and tissue substitutes approved by the FDA and the Centers for Medicare & Medicaid Services have been established for individuals meeting specified selection criteria. They may be useful therapeutic options when indicated.
Human tissue products are subject to the rules and regulations of banked human tissue by the American Association of Tissue Banks, or AATB, and have been established for individuals meeting specified selection criteria. They may be useful therapeutic options when indicated.
Notes:
- Non-human tissues qualify for FDA approval.
- Human tissues are governed by the American Tissue Bank and don’t qualify for FDA approval.
Updates were made to criteria and procedure code A2021 has been made payable, effective Nov. 1, 2023.
Inclusions:
The following skin and tissue substitutes are considered established when used according to the FDA approval. This list may not be all-inclusive:
- Apligraft®
- Apis®
- Atlas Wound Matrix
- Biobrane®
- Bio-conneKt® Wound Care Matrix
- BTM Wound Dressing (aka NovoSorb® BTM)
- Cytal® Burn Matrix
- Cytal® MicroMatrix™
- CytalTM Wound Matrix (formerly MatriStem)
- Cytal® Wound Sheet
- Derma-Gide (aka Geistlich Derma-Gide™)
- Dermagraft®
- Endoform Dermal Template™
- Epicel® has FDA humanitarian device approval
- E-Z Derm™
- Helicoll™
- Hyalomatrix®
- InnovaMatrix™ (also known as InnovaMatrix AC)
- InnovaMatrix™ FS
- Integra® Bilayer Matrix
- Integra® Dermal Regeneration Template
- Integra® Flowable Wound Matrix
- Intregra® Matrix Wound Dressing (formerly known as Avagen)
- Keratec Wound Dressings (Kermatrix®)
- Keratec Keragel
- Keraderm
- Kerafoam
- Kerecis Limited MariGen Wound Extra
- Kerecis™ Omega3 Wound (formerly known as MeriGen)
- MediSkin®
- Microlyte® Ag
- MicroMatrix®
- Mirragen™
- NeoMatriX® Wound Matrix
- Oasis® Burn Matrix
- Oasis® Ultra Tri-Layer Wound Matrix
- Oasis® Wound Matrix
- Ologen™ Collagen Matrix
- Omeza® Collagen Matrix
- OrCel®
- PELNACTM Bilayer Wound Matrix
- Permacol™ (Covidien)
- PermeaDerm B
- PermeaDerm C
- PermeaDerm Glove
- Phoenix™ Wound Matrix
- PriMatrix™ Dermal Repair Scaffold
- Puracol® and Puracol® Plus Collagen Wound Dressings
- PuraPly Antimicrobial Wound Matrix (PuraPly AM; formerly known as FortaDerm AM)
- PuraPly Micronized Wound Matrix (PuraPly MZ; formerly known as FortaDerm)
- Restrata®
- Strattice™
- Suprathel®
- SupraSDRM Biodegradable Matrix Wound Dressing
- SurgiMend®
- Symphony™
- Talymed™
- TenoGlide™
- TransCyte®
- XCelliStem® Wound Powder
Breast reconstructive surgery using allogeneic acellular dermal matrix productsa (including each of the following: AlloDerm®, AlloMend®, Cortiva®, [AlloMax™], DermACELL™, DermaMatrix™, FlexHD®, FlexHD® Pliable™, Graftjacket®) are considered established when one of the following are met:
- There is insufficient tissue expander or implant coverage by the pectoralis major muscle and additional coverage is required.
- There is viable but compromised or thin postmastectomy skin flaps that are at risk of dehiscence or necrosis.
- The inframammary fold and lateral mammary folds have been undermined during mastectomy and reestablishment of these landmarks is needed.
Note: Various acellular dermal matrix products used in breast reconstruction have similar efficacy. The products listed are those that have been identified for use in breast reconstruction. Additional acellular dermal matrix products may become available for this indication.
Treatment of chronic, noninfected, full-thickness diabetic lower extremity ulcers is established when using the following tissue-engineered skin substitutes:
- AlloPatch® a
- Apligraft® b
- Dermagraft® b
- GraftJacket® Regenerative Tissue Matrix-Ulcer Repair
- Integra®, Omnigraft™ Dermal Regeneration Matrix (also known as Omnigraft™) and Integra Flowable Wound Matrix
- Theraskin®
Treatment of chronic, noninfected, partial- or full-thickness lower-extremity skin ulcers due to venous insufficiency, which haven’t adequately responded following a one-month period of conventional user therapy is established when using the following tissue-engineered skin substitutes:
- Aplifraf® b
- Oasis™ Wound Matrixc
- Theraskin®
OrCel™ is considered established when all of the following criteria are met:
- Used for the treatment of dystrophic epidermolysis bullosa.
- Used for the treatment of mitten-hand deformity.
- Standard would therapy has failed.
- Provided in accordance with the humanitarian device exemption, or HDE, specifications of the FDA.
The following skin and tissue products and substitutes are considered established for use in the treatment of second- and third-degree burns:
- Alloderm
- Epicel® (for the treatment of deep dermal or full-thickness burns comprising a total body surface area ≥30% when provided in accordance with the HDE specifications of the FDA)d
- Integra® Dermal Regeneration Templateb
aBanked human tissue
bFDA premarket approval
cFDA 510(k) clearance
dFDA approved under an HDE
Exclusions:
All other uses of bioengineered skin and soft tissue substitutes listed above are excluded unless they meet one of the following criteria:
- FDA approval and provided in accordance with the FDA guidelines
- Covered by CMS
All other skin and soft tissue substitutes, including, but not limited to:
- ACell® UBM Hydrated/Lyophilized Wound Dressing
- AlloSkin™
- AlloSkin™ RT
- Aongen™ Collagen Matrix
- Architect® ECM, PX, FX
- ArthroFlex™ (Flex Graft)
- AxoGuard® Nerve Protector (AxoGen)
- BellaCell HD or SureDerm®
- CollaCare®
- CollaCare® Dental
- Collagen Wound Dressing (Oasis Research)
- CollaGUARD®
- CollaMend™
- CollaWound™
- Coll-e-Derm
- Collexa®
- Collieva®
- Conexa™
- Coreleader Colla-Pad
- CorMatrix®
- Cymetra™ (Micronized AlloDerm™)
- Dermadapt™ Wound Dressing
- DermaPure™
- DermaSpan™
- DressSkin
- Durepair Regeneration Matrix®
- ENDURAGen™
- Excellagen
- ExpressGraft™
- FlexiGraft®
- FlowerDerm®
- GammaGraft
- hMatrix®
- InteguPly®
- Kerecis omega3 marigen
- Keroxx™
- MatriDerm®
- Matrix HD™
- MemoDerm™
- Microderm® biologic wound matrix
- Miroderm®
- MyOwn Skin™
- NeoForm™
- Progenamatrix™
- PuraPly XT
- Puros® Dermis
- RegenePro™
- Repliform®
- Repriza™
- SkinTE™
- SlimpliDerm®
- StrataGraft®
- TenSIX™ Acellular Dermal Matrix
- TissueMend
- TheraForm™ Standard/Sheet
- TruSkin™
- Veritas® Collagen Matrix
- XCM Biologic® Tissue Matrix
- XenMatrix™ AB
Systems, kits or devices used to prepare or construct skin or tissue substitutes including:
- Ac5 advanced wound system
- Recell® Autologous Cell Harvesting Device
|
C9399, J3490, J3590, J9999 |
Basic benefit and medical policy
Avzivi (bevacizumab-tnjn)
Avzivi (bevacizumab-tnjn) is considered established, effective Dec. 6, 2023.
Avzivi is a vascular endothelial growth factor inhibitor indicated for the treatment of:
- Metastatic colorectal cancer, in combination with intravenous fluorouracil-based chemotherapy for first- or second-line treatment.
- Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy for second-line treatment in patients who have progressed on a first-line bevacizumab product-containing regimen.
Limitations of use
- Avzivi isn’t indicated for adjuvant treatment of colon cancer.
- Unresectable, locally advanced, recurrent or metastatic non-squamous non-small cell lung cancer, in combination with carboplatin and paclitaxel for first-line treatment.
- Recurrent glioblastoma in adults.
- Metastatic renal cell carcinoma in combination with interferon alfa.
- Persistent, recurrent or metastatic cervical cancer, in combination with paclitaxel and cisplatin, or paclitaxel and topotecan.
- Epithelial ovarian, fallopian tube or primary peritoneal cancer in combination with paclitaxel, pegylated liposomal doxorubicin, or topotecan for platinum-resistant recurrent disease who received no more than two prior chemotherapy regimens.
Dosage and administration
Withhold for at least 28 days before elective surgery. Don’t administer Avzivi for 28 days following major surgery and until adequate wound healing.
Metastatic colorectal cancer:
- 5 mg/kg every two weeks with bolus-IFL
- 10 mg/kg every two weeks with Folfox4
- 5 mg/ kg every two weeks or 7.5 mg/kg every three weeks with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy after progression on a first-line bevacizumab product containing regimen
First-line non-squamous non-small cell lung cancer:
15 mg/kg every three weeks with carboplatin and paclitaxel
Recurrent glioblastoma:
10 mg/kg every two weeks
Metastatic renal cell carcinoma:
10 mg/kg every two weeks with interferon alfa
Persistent, recurrent or metastatic cervical cancer:
15 mg/kg every three weeks with paclitaxel and cisplatin, or paclitaxel and topotecan
Platinum-resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer:
- 10 mg/kg every two weeks with paclitaxel, pegylated liposomal doxorubicin or topotecan given every week
- 15 mg/kg every three weeks with topotecan given every three weeks
Administer as an intravenous infusion after dilution.
Dosage forms and strengths
Injection: 100 mg/4 mL (25 mg/mL) or 400 mg/16 mL (25 mg/mL) in a single-dose vial.
This drug isn’t a benefit for URMBT.
|
C9399, J3490, J3590, J9999 |
Basic benefit and medical policy
Focinvez (fosaprepitant)
Focinvez (fosaprepitant) is considered established when criteria are met, effective Aug. 24, 2023.
Focinvez is a substance P/neurokinin-1, or NK1, receptor antagonist, indicated in adults and pediatric patients 6 months of age and older, in combination with other antiemetic agents, for the prevention of:
- Acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy, or HEC, including high-dose cisplatin.
- Delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy, or MEC.
Limitations of use
Focinvez hasn’t been studied for the treatment of established nausea and vomiting.
Dosage and administration
Recommended adult dosage:
- Focinvez 150 mg on Day 1 as an intravenous infusion over 20 to 30 minutes.
- Complete the infusion approximately 30 minutes before chemotherapy.
Recommended dosage for pediatric patients (6 months to 17 years) weighing at least 6 kg:
- Single dose chemotherapy regimens: Single dose of Focinvez on Day 1.
- Single or multi-day chemotherapy regimens: Three-day regimen of Focinvez on Day 1 and aprepitant capsules or aprepitant for oral suspension on Days 2 and 3.
- Administer FOCINVEZ through a central venous catheter on Day 1 as an intravenous infusion over 30 minutes (12 years to 17 years) or 60 minutes (6 months to less than 12 years).
- Complete the infusion approximately 30 minutes before chemotherapy.
Dosage forms and strengths
Injection: 150 mg/50 mL (3 mg/mL) of fosaprepitant, in a single-dose vial.
Focinvez (fosaprepitant) isn’t a benefit for URMBT. |
C9399, J3490, J3590, J9999 |
Basic benefit and medical policy
Lyfgenia (lovotibeglogene autotemcel)
Effective Dec. 8, 2023, Lyfgenia (lovotibeglogene autotemcel) is covered for its FDA-approved indications.
Coverage of Lyfgenia (lovotibeglogene autotemcel) is provided when all the following are met:
- FDA-approved indication
- FDA-approved age
- Prescribing by or in consultation with a hematologist
- Genetic test confirming a diagnosis of sickle cell disease
- Must not be diagnosed with sickle β-thalassemia
- Must have experienced at least four severe vaso-occlusive crises in the past 24 months
- Trial and failure, contraindication or intolerance to hydroxyurea
- Must not have any of the following:
- Positive presence of HIV-1 or HIV-2, hepatitis B or hepatitis C
- Inadequate bone marrow function, as defined by an absolute neutrophil count of less than 1000/μL or less than 500/μL for patient taking hydroxyurea or a platelet count less than 120,000/μL without hypersplenism
- Advanced liver disease defined as AST, ALT or total bilirubin greater than three times the upper limit of normal
- Prior treatment with an allogenic stem cell transplant
- Prior or current malignancy or immunodeficiency disorder
- Must not have received prior treatment with any gene therapy for sickle cell disease or are being considered for treatment with any other gene therapy for sickle cell disease
- Trial and failure, intolerance or a contraindication to the preferred products as specified in the Blue Cross Blue Shield of Michigan or Blue Care Network medical utilization management drug list
Quantity limitations, authorization period and renewal criteria:
- Quantity limits: Align with FDA-recommended dosing
- Initial authorization period: Three months
- Renewal criteria: No renewal allowed, one infusion per lifetime
This drug isn’t a benefit for URMBT. |
J3490, J3590 |
Basic benefit and medical policy
Daxxify (daxibotulinumotoxina-lanm)
Effective Aug. 11, 2023, the FDA indications have been updated for Daxxify (daxibotulinumotoxina-lanm) to include the treatment of cervical dystonia in adult patients.
Dosage and administration
Cervical dystonia: The recommended dose is 125 units to 250 units given intramuscularly as a divided dose among affected muscles.
Daxxify (daxibotulinumotoxina-lanm) isn’t a benefit for URMBT.
|
J3490, J3590 |
Basic benefit and medical policy
Omvoh (mirikizumab-mrkz)
Effective Oct. 26, 2023, Omvoh (mirikizumab-mrkz) is covered for its FDA-approved indications.
Coverage of Omvoh (mirikizumab-mrkz) is provided when all the following are met:
- FDA-approved indication
- FDA-approved age
- Treatment with an adequate course of conventional therapy (such as steroids for seven days, immunomodulators such as azathioprine for at least two months) has been ineffective or is contraindicated or not tolerated.
- Not to be used in combination with biologic therapies or targeted disease-modifying anti-rheumatic drugs, known as DMARDs.
- Trial and failure, contraindication or intolerance to the preferred drugs as listed in Blue Cross’ or BCN’s utilization management medical drug list
Quantity limitations, authorization period and renewal criteria:
- Quantity limits: Align with FDA-recommended dosing
- Initial authorization period: One year at a time
- Renewal criteria: Clinical documentation must be provided to confirm that current criteria are met and that the medication is providing clinical benefit.
This drug isn’t a benefit for URMBT. |
J3490, J3590 |
Basic benefit and medical policy
Rivfloza (nedosiran)
Rivfloza (nedosiran) is considered established when criteria are met, effective Sept. 29, 2023.
Coverage of Rivfloza (nedosiran) is provided when all the criteria are met:
- Diagnosis of primary hyperoxaluria type 1, or PH1, confirmed by genetic testing of the AGXT mutation.
- FDA-approved age
- Patient has an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2.
- Patient doesn’t have a history of kidney or liver transplant.
- Trial and failure to at least three months, contraindication or intolerance to a course of high-dose vitamin B-6 therapy.
- The member will self-administer Rivfloza unless clinically unable to do so.
- Won’t be used in combination with Oxlumo.
- Trial and failure, contraindication or intolerance to the preferred drugs as listed in Blue Cross Blue Shield of Michigan’s or Blue Care Network’s utilization management medical drug list or Blue Cross’ or BCN’s prior authorization and step therapy documents.
Quantity limitations, authorization period and renewal criteria
- Quantity limits: Align with FDA-recommended dosing
- Authorization period: One year at a time
- Renewal criteria: Clinical documentation must be provided to confirm that current criteria are met and that the medication is providing clinical benefit.
This drug isn’t a benefit for URMBT. |
J3490, J3590 |
Basic benefit and medical policy
Tofidence (tocilizumab-bavi)
Effective Sept. 29, 2023, Tofidence (tocilizumab-bavi) is covered for its FDA-approved indications.
Coverage of Tofidence (tocilizumab-bavi) is provided when all the following are met:
- FDA-approved indication
- FDA-approved age
- Diagnosis of rheumatoid arthritis: Trial and failure of at least three months of one disease-modifying anti-rheumatic agent, or DMARD, unless contraindicated or not tolerated. Examples include methotrexate, hydroxychloroquine, leflunomide and sulfasalazine.
- Diagnosis of polyarticular juvenile idiopathic arthritis, or pJIA: Trial and failure of at least three months of one DMARD unless contraindicated or not tolerated. Examples include methotrexate and leflunomide.
- Diagnosis of Still’s disease, including systemic juvenile idiopathic arthritis, or sJIA, and adult-onset Still’s disease, or AOSD: Trial and treatment failure with one of the following: glucocorticoids or NSAIDs.
- Diagnosis of cytokine release syndrome, or CRS: Prescribed by or in consultation with an oncologist. Severe or life-threatening CRS associated with chimeric antigen receptor, or CAR-T cell therapy.
- Diagnosis of giant cell arteritis, or GCA.
- Diagnosis of systemic sclerosis-associated interstitial lung disease, or SSc-ILD: Inadequate response to (as evidenced by disease progression, e.g., worsening of pulmonary function) or not a candidate for either mycophenolate mofetil or cyclophosphamide.
- Not to be used in combination with other biologics or other targeted DMARDs.
- Coverage will be provided for biosimilar products for FDA-labeled indications of the innovator product when criteria are met.
- Trial and failure of the preferred products as specified in the Blue Cross Blue Shield of Michigan and Blue Care Network utilization management medical drug list or the Blue Cross and BCN prior authorization and step therapy documents.
Quantity limitations, authorization period and renewal criteria
Quantity limits:
Align with FDA-recommended dosing
Initial authorization period:
RA, pJIA, sJIA, GCA, AOSD, SSc-ILD: One year at a time
CRS: 60 days
Renewal criteria:
RA, pJIA, sJIA, GCA, AOSD, SSc-ILD: Clinical documentation must be provided to confirm that current criteria are met and that the medication is providing clinical benefit.
CRS: Not applicable as no further authorization will be provided.
This drug isn’t a benefit for URMBT.
|
None of the information included in this billing chart is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.

Blue Cross changing practitioner fees July 1
Blue Cross Blue Shield of Michigan will change practitioner fees for services with dates of service on or after July 1, 2024. This change applies to services provided to our Traditional, TRUST and Blue Preferred Plus℠ members, regardless of the customer group.
Blue Cross will use the 2024 Medicare resource-based relative value scale for most relative value unit priced procedures for dates of service on and after July 1. Most fees are currently priced using the 2023 values.
The Blue Cross overall fee update includes base fee adjustments and value-based reimbursement. Due to significant changes in relative value unit valuations, it’s best to review the fee schedule to view the effect on an individual code or group of codes.
Fee schedules effective July 1 will be available on the Provider Resources site through our provider portal on Availity® Essentials. To find the fee schedules:
- Log in to availity.com.**
- Click on Payer Spaces on the menu bar.
- Click on the BCBSM and BCN logo.
- Scroll down and then click on Secure Provider Resources (Blue Cross and BCN).
- Click on the Fee Schedule drop-down menu and then click on Blue Cross Professional Fee Schedules.
Only claims submitted with dates of service on or after July 1 will be reimbursed at the new fees.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Availity is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services.
Reminder: Confirm data every 90 days, attest in CAQH every 120 days
What you need to know
To remain listed in Blue Cross Blue Shield of Michigan’s provider directories, including Find a Doctor, health care providers must re-attest every 120 days in CAQH.
Have you confirmed your data within the past 90 days and attested in CAQH within the past 120 days? Health care providers are required to confirm the following data elements every 90 days: name, specialty, address, phone number and digital contact information. Providers are also required to re-attest every 120 days for all other data elements, including credentialing, licensing and demographics.
If providers don’t re-attest with CAQH every 120 days, they won’t be included in Blue Cross Blue Shield of Michigan’s provider directories, including our Find a Doctor search tool. Your credentialing status will end if you fail to re-attest, and you’ll need to reapply.
It’s important to attest with CAQH to:
- Ensure your affiliation with Blue Cross isn’t interrupted.
- Keep your contact information up to date.
- Make sure claims payments aren’t interrupted.
Providers who practice at an office location or practice exclusively in an inpatient hospital setting also need to perform this attestation. If you’re practicing exclusively in an inpatient hospital setting, you must indicate that on your CAQH application. This information is used to determine whether full credentialing is required.
CAQH is a nonprofit alliance of health plans and trade associations focused on simplifying health care administration. Blue Cross uses CAQH to gather and coordinate our practitioner credentialing information.
All health care practitioners, including hospital-based providers, need to be registered with CAQH.
If you have questions about CAQH, call the help desk at 1-888-599-1771, or go to CAQH.org.**
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Blue Cross enhances Medicare Plus Blue attribution model by adding PCP Select option
Blue Cross Blue Shield of Michigan is adding a new option to the retrospective claims-based Medicare Plus Blue℠ attribution model. PCP Select will give Medicare Plus Blue members the option to select primary care providers in their member portal or mobile phone applications. Members who select primary care providers in the portal will be attributed based on the primary care providers they selected, rather than on the standard claims model.
PCP Select should be available to members beginning May 1, 2024. Medicare Plus Blue members who choose not to select a primary care provider will continue to be attributed based on the standard claims model. Selecting a primary care provider in the member portal won’t limit which providers a member can see for care, nor will it affect a member’s benefits or claims payments. Instead, it will allow for quicker and more efficient attribution, which will improve data sharing and financial operations.
What you need to know
- Members will be notified of this option when they log in to their member portal accounts after PCP Select is available.
- Instructions on the portal will advise members to select a primary care provider only after they’ve established care with that provider (for example, have had visits with the provider and plan to continue seeing the provider for primary care). We encourage this, but it’s not a requirement.
- If members need help with the selection process, they can call the Customer Service number on the back of their member ID card.
If providers have questions about this Medicare Plus Blue attribution enhancement, call Provider Inquiry at 1-866-309-1719.
New patient experience resources for practices
Blue Cross Blue Shield of Michigan has added new patient experience resources that focus on strategies for communicating with patients to provide a great patient experience. They include webinars and additional content to our on-demand library.
In April, we’ll be presenting a three-part webinar series, “Improving health outcomes for older adults,” to help physicians and clinical staff navigate the complexities of discussing potentially sensitive issues with older patients. It’ll include topics such as urinary incontinence, fall risk and mental health — concerns that can have a significant effect on patients’ health, longevity and well-being. Each session will give strategies to enhance patient communication and foster open dialog that can lead to improved care and outcomes for older adults.
Register for these virtual webinars by visiting the Upcoming Webinars page of the Blue Cross Patient Experience site. All sessions will be from noon to 12:45 p.m.
Part 1: April 16, 2024 — We’ll focus on ensuring effective care through conversations with patients about urinary incontinence, physical activity and fall risk, along with a brief background on the Health Outcomes Survey.
Part 2: April 18, 2024 — Participants will learn strategies to discuss mental and physical health with older adults. The session will also discuss using patient-centered planning to improve or maintain patients’ health.
Part 3: April 23, 2024 — Learn about the barriers and anxiety older patients have to broaching discussions about sensitive topics, such as memory problems and advanced care planning. We’ll explore providers’ apprehension to bring up sensitive topics, and share tips to relieve patients’ anxiety, ways to build trust and strategies providers can use to overcome their own anxieties toward sensitive conversations.
Recordings will be available on demand at the Patient Experience site after the live sessions. Physicians who attend this 90-minute total workshop can receive CME credits. CEU’s are also available for medical assistants.
Check out our on-demand library
Visit the On-Demand Content page of the Patient Experience site to view the latest educational resources.
- The “Managing Challenging Patient Interactions” webinar provides strategies for health care professionals to manage upset or frustrated patients or caregivers. It covers tactics to prevent challenging situations, tips to stay calm and de-escalation strategies. “Managing Challenging Patient Interactions” is also available as an in-office training session.
- Over the past few years, many physician practices have adopted the use of telehealth to increase access for patients and provide greater flexibility in scheduling. To help practices ensure their virtual visits provide the same patient experience as in-office visits, we’ve developed the e-learning series titled “Telehealth – Processes to maintain a great patient experience.” Two modules are available:
- A module for clinicians focused on patient-centered care and communication tips (15 minutes)
- A module for office managers or leads with tips to ensure telehealth appointments are successful (15 minutes)
For these and other on-demand resources, CME credits are available for physicians and CEU’s are available for medical assistants.
For more information, email PatientExperience@bcbsm.com.
Reminder: How billing works for care authorized by VA at non-VA facilities for FEP members
Veteran Administration benefits are coordinated with Blue Cross and Blue Shield Federal Employee Program® benefits.
When a VA facility authorizes care by a non-VA health care provider, the VA facility is considered the billing provider, and the non-VA provider is considered the performing provider. The VA, as the billing provider, is responsible for filing the member’s claim for the FEP® benefit to the local plan. The non-VA provider is reimbursed by the VA facility.
Ambulance providers should use appropriate modifiers for transportation to or from crisis stabilization units
Blue Cross Blue Shield of Michigan and Blue Care Network recognize crisis stabilization units as therapeutic sites. Crisis stabilization units provide immediate behavioral health support and treatment services in a therapeutic setting to individuals in acute behavioral health distress who require urgent care beyond what an outpatient behavioral health provider can provide. Crisis stabilization units can help to deescalate a person’s level of distress, prevent, or treat a behavioral health crisis, and reduce acute symptoms of a behavioral health condition.
Diagnostic or therapeutic sites are recognized by the origin or destination with a “D.” Effective April 1, 2024, ambulance providers should report the following modifiers for Basic Life Support, or BLS, emergency ambulance services with an origin or destination for a crisis stabilization unit:
- DH ̶ From the crisis stabilization unit to the hospital
- HD ̶ From the hospital to the crisis stabilization unit
- RD ̶ From the residence to the crisis stabilization unit
- SD ̶ From scene of an accident to the crisis stabilization unit
Starting April 1, additional preferred product required for Soliris, Ultomiris for most commercial members
For dates of service on or after April 1, 2024, step therapy requirements will change for Soliris® (eculizumab), HCPCS code J1300, and Ultomiris® (ravulizumab), HCPCS code J1303.
Preferred products for Soliris and Ultomiris |
Before April 1, 2024 |
On or after April 1, 2024 |
Members must try and fail:
|
Members must try and fail both:
- Rystiggo®
- Either Vyvgart or Vyvgart Hytrulo
|
This change affects Blue Cross Shield of Michigan commercial members and Blue Care Network commercial members.
By April 1, we’ll update the Blue Cross and BCN utilization management medical drug list to reflect the new preferred drugs.
The drugs discussed above continue to require prior authorization through the NovoLogix® online tool.
Some Blue Cross commercial groups aren’t subject to these requirements
For Blue Cross commercial groups, this prior authorization requirement applies only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list.
Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program.
Additional information
For more information about medical benefit drugs, see the following pages on ereferrals.bcbsm.com:
Prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
We’ve changed how we manage Entyvio SC, Omvoh SC
On March 1, 2024, Blue Cross Blue Shield of Michigan and Blue Care Network changed how we manage the following drugs for our Medicare Plus Blue℠ and BCN Advantage℠ members:
- Entyvio® SC (vedolizumab), HCPCS code J3590
- Omvoh™ SC (mirikizumab-mrkz), HCPCS code J3590
Note: This change doesn’t affect Entyvio IV, HCPCS code J3380, or Omvoh IV, HCPCS code J3590, which will continue to be managed as part of members’ Part B medical benefits. These drugs continue to require prior authorization through the NovoLogix® web tool.
What changed on March 1
On March 1, Medicare Plus Blue and BCN Advantage members who previously received Entyvio SC or Omvoh SC under the Part B medical benefit were required to continue their treatments under their Part D pharmacy benefits.
We made this change because these therapies can be safely and conveniently self-administered in the home; the Centers for Medicare & Medicaid Services, or CMS, has added these drugs to the Self-Administered Drug Exclusion List: (SAD List).**
As a result:
- These drugs are no longer covered when administered by a doctor or other health care professional under the Part B medical benefit.
- Entyvio SC and Omvoh SC aren’t included in our Medicare Advantage Part D formularies, but providers can request prior authorization for them as exceptions. (See the “How to submit prior authorization requests for Entyvio SC and Omvoh SC” section below.)
- Your patients can obtain these medications at pharmacies that dispense specialty drugs. They can also obtain these drugs from AllianceRx Walgreens Pharmacy through mail order or pickup at a Walgreens retail pharmacy.
- For members who don’t have Part D pharmacy benefits through Blue Cross or BCN, providers need to work with the pharmacy vendor that provides each member’s Part D coverage.
How to submit prior authorization requests for Entyvio SC and Omvoh SC
For members who have Part D pharmacy benefits through Medicare Plus Blue or BCN Advantage, providers need to submit prior authorization requests for Entyvio SC and Omvoh SC as follows:
List of requirements
For a full list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue and BCN Advantage members.
Authorization isn't a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Additional cardiology codes will require prior authorization for commercial members, starting May 11
For dates of service on or after May 11, 2024, these additional cardiology codes will require prior authorization by Carelon Medical Benefits Management for Blue Cross Blue Shield of Michigan and Blue Care Network commercial members:
Starting May 11, Carelon will use the Blue Cross and BCN medical policy titled Leadless Cardiac Pacemakers as the criteria for making determinations on prior authorization requests. To access this policy, open the Medical Policy Router Search page on bcbsm.com, enter the name of the policy in the Policy/Topic Keyword field and press Enter.
You’ll be able to access Carelon’s clinical criteria for these procedures, when available, on the Current Cardiology Guidelines** page of the Carelon website.
Additional information
By May 11, we’ll update this document to include the previously mentioned codes.
For more resources related to the cardiology procedures that require prior authorization, refer to these webpages at ereferrals.bcbsm.com:
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage prior authorizations for select services.
We’ve posted our 2024 HEDIS tip sheets
Our 2024 HEDIS® tip sheets are posted in the Clinical Quality section of Secure Provider Resources on our provider portal. Each year, we update and post our HEDIS and Star measure tip sheets. The Star measure tip sheets were posted earlier this year.
About our tip sheets
- HEDIS tip sheets are developed to assist health care providers and their staff in efforts to improve overall health care quality and prevent or control diseases and chronic conditions. HEDIS is one of the most widely used performance improvement tools in the U.S.
- Star tip sheetshighlight select measures in the Medicare Star Ratings program. Most of the measures highlighted in our Star tip sheetsare also HEDIS measures.
Accessing the tip sheets
To access our tip sheets:
- Log in to our provider portal at availity.com.**
- Under Payer Spaces, click on the BCBSM and BCN logo.
- Click on Secure Provider Resources (Blue Cross and BCN) under the Resources tab.
- Under the Member Care tab, click on Clinical Quality Tip Sheets.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
HEDIS®, which stands for Healthcare Effectiveness Data and Information Set, is a registered trademark of the National Committee for Quality Assurance, or NCQA.
Webinars for physicians, coders focus on risk adjustment, coding
Beginning in April 2024, we’ll offer webinars about documentation and coding of common challenging diagnoses. These live, lunchtime educational sessions will also include an opportunity to ask questions.
Below is our schedule and the tentative topics for the sessions. All sessions start at noon Eastern time and generally last for 30 minutes. Register for the session that best works with your schedule on the provider training website.
Session date |
Topic |
April 17 |
HCC and Risk Adjustment Updates |
May 22 |
Medical Record Documentation and MEAT |
June 26 |
Orthopedic and Sports Medicine Coding Tips |
July 10 |
Diabetes and Weight Management Coding Tips |
Aug. 21 |
Cardiovascular Disease and Vascular Surgery Coding Tips |
Sept. 18 |
Neurosurgery, Dementia and Cognitive Impairment Coding Tips |
Oct. 2 |
ICD-10-CM Updates |
Nov. 13 |
Oncology Coding Tips |
Dec. 11 |
CPT Updates 2025 |
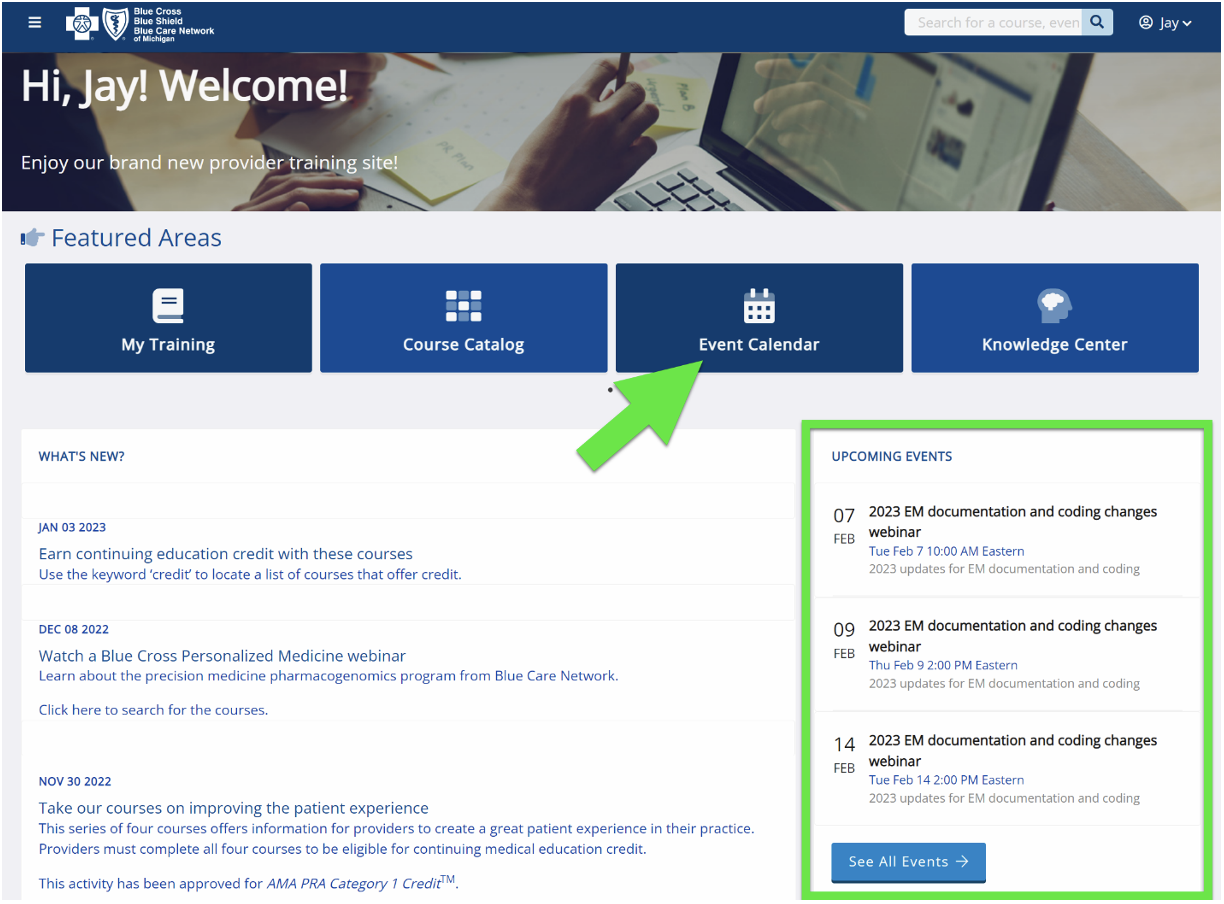
Provider training website access
Provider portal users with an Availity® Essentials account can access the provider training website by logging in to availity.com,** clicking on Payer Space in the top menu bar and then clicking on the BCBSM and BCN logo. Then click on the Applications tab, scroll down to the Provider Training Site tile and click on it.
You can also directly access the training website if you don’t have a provider portal account: Provider training website.
After logging in to the provider training website, look in Event Calendar to sign up for your desired session. You can also quickly search for all the sessions with the keyword “lunchtime" and then look under the results for Events.
Questions?
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
Register now for 2024 virtual provider symposium sessions
This year’s virtual provider symposiums focusing on quality measures, documentation and coding guidelines will start in May. Registration is now open on the provider training website. Physicians, physician assistants, nurse practitioners, nurses and coders can receive continuing education credits for attending.
Once you’re logged in to the provider training site, open the event calendar to sign up for any of the sessions listed below. You can also quickly search for all the sessions with the keyword “symposium” and then look under the results for Events.
All Star Performance-HEDIS® / Star Rating Measure Overview: For physicians and office staff responsible for closing gaps in care related to quality adult measures
Session |
Date |
Time |
All Star Performance- HEDIS® / Star Rating Measure Overview |
May 9 |
9 to 10 a.m. |
All Star Performance- HEDIS® / Star Rating Measure Overview |
May 15 |
9 to 10 a.m. |
All Star Performance- HEDIS® / Star Rating Measure Overview |
May 23 |
2 to 3 p.m. |
All Star Performance- HEDIS® / Star Rating Measure Overview |
June 4 |
2 to 3 p.m. |
Coding and Documentation Tips for 2024 and Beyond: For physicians, coders, billers and administrative staff
Session |
Date |
Time |
Let’s Talk Coding: Coding and Documentation Tips for 2024 and Beyond |
May 7 |
11 a.m. to noon |
Let’s Talk Coding: Coding and Documentation Tips for 2024 and Beyond |
May 16 |
3 to 4 p.m. |
Let’s Talk Coding: Coding and Documentation Tips for 2024 and Beyond |
May 21 |
9 to 10 a.m. |
Let’s Talk Coding: Coding and Documentation Tips for 2024 and Beyond |
June 6 |
11 a.m. to noon |
Provider training website access
Provider portal users with an Availity® Essentials account can access the provider training website by logging in to availity.com,** clicking on Payer Space in the top menu bar and then clicking on the BCBSM and BCN logo. Then click on the Applications tab, scroll down to the Provider Training Site tile and click on it.
You can also directly access the training website if you don’t have a provider portal account.
Questions?
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
HEDIS® is a registered trademark of the National Committee for Quality Assurance.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
Changes to Gastric stimulation and Breast reconstruction questionnaires in e-referral system
On Feb. 25, 2024, we updated questionnaires in the e-referral system.
We also updated the corresponding preview questionnaires in the Authorization criteria and preview questionnaires document on the ereferrals.bcbsm.com website.
As a reminder, we use our authorization criteria, our medical policies and your answers to the questionnaires in the e-referral system when making utilization management determinations on your prior authorization requests.
Updated questionnaires
We updated the following questionnaires in the e-referral system:
Questionnaire |
Opens for |
Updates |
Gastric stimulation |
- Medicare Plus Blue℠
- BCN commercial
- BCN Advantage℠
|
Removed a question. |
Breast reconstruction |
|
Updated a question. |
Preview questionnaires
Preview questionnaires show the questions you’ll need to answer in the e-referral system so you can prepare your answers ahead of time.
To find the preview questionnaires, see the document titled Authorization criteria and preview questionnaires.
You can access this document by going to ereferrals.bcbsm.com and doing the following:
- For Medicare Plus Blue: Click on Blue Cross and then click on Prior Authorization. Scroll down and look under the Authorization information for Medicare Plus Blue members heading.
- For BCN: Click on BCN and then click on Prior Authorization & Plan Notification. Scroll down and look under the Authorization criteria and preview questionnaires for select services heading.
Authorization criteria and medical policies
The Authorization criteria and preview questionnaires document explains how to access the pertinent authorization criteria and medical policies.
Resources for prenatal care, childhood immunizations
This is part of an ongoing series of articles focusing on the tools and resources available to help FEP members manage their health.
Early prenatal care and childhood immunizations support a healthy pregnancy and start to a child’s life. Here are some resources for prenatal care and childhood immunizations.
Prenatal care
Because of the importance of early prenatal care, the National Committee for Quality Assurance created a Healthcare Effectiveness Data and Information Set, or HEDIS®, measure. To help health care providers understand the measure and how to report compliance, Blue Cross Blue Shield of Michigan created a prenatal care HEDIS tip sheet.
The Blue Cross and Blue Shield Federal Employee Program® developed the Your Pregnancy To-Do List flyer to help its members prepare for a healthy and safe pregnancy.
Childhood immunizations
To help babies have a healthy start to life, the American Academy of Pediatrics recommends specific vaccines before the age of 2. The Childhood Immunizations Status (CIS) HEDIS measure helps support childhood immunizations, and Blue Cross Blue Shield of Michigan created a CIS tip sheet to help providers understand the measure and report compliance.
The Federal Employee Program also developed the Good Health Starts Early Well-Child Quick Reference Guide for its members. It includes the recommended vaccine schedule from birth to 18, along with other health information.
For information on FEP benefits, members and providers can call Customer Service at 1-800-482-3600 or go online to fepblue.org.
HEDIS® is a registered trademark of the National Committee for Quality Assurance.

New patient experience resources for practices
Blue Cross Blue Shield of Michigan has added new patient experience resources that focus on strategies for communicating with patients to provide a great patient experience. They include webinars and additional content to our on-demand library.
In April, we’ll be presenting a three-part webinar series, “Improving health outcomes for older adults,” to help physicians and clinical staff navigate the complexities of discussing potentially sensitive issues with older patients. It’ll include topics such as urinary incontinence, fall risk and mental health — concerns that can have a significant effect on patients’ health, longevity and well-being. Each session will give strategies to enhance patient communication and foster open dialog that can lead to improved care and outcomes for older adults.
Register for these virtual webinars by visiting the Upcoming Webinars page of the Blue Cross Patient Experience site. All sessions will be from noon to 12:45 p.m.
Part 1: April 16, 2024 — We’ll focus on ensuring effective care through conversations with patients about urinary incontinence, physical activity and fall risk, along with a brief background on the Health Outcomes Survey.
Part 2: April 18, 2024 — Participants will learn strategies to discuss mental and physical health with older adults. The session will also discuss using patient-centered planning to improve or maintain patients’ health.
Part 3: April 23, 2024 — Learn about the barriers and anxiety older patients have to broaching discussions about sensitive topics, such as memory problems and advanced care planning. We’ll explore providers’ apprehension to bring up sensitive topics, and share tips to relieve patients’ anxiety, ways to build trust and strategies providers can use to overcome their own anxieties toward sensitive conversations.
Recordings will be available on demand at the Patient Experience site after the live sessions. Physicians who attend this 90-minute total workshop can receive CME credits. CEU’s are also available for medical assistants.
Check out our on-demand library
Visit the On-Demand Content page of the Patient Experience site to view the latest educational resources.
- The “Managing Challenging Patient Interactions” webinar provides strategies for health care professionals to manage upset or frustrated patients or caregivers. It covers tactics to prevent challenging situations, tips to stay calm and de-escalation strategies. “Managing Challenging Patient Interactions” is also available as an in-office training session.
- Over the past few years, many physician practices have adopted the use of telehealth to increase access for patients and provide greater flexibility in scheduling. To help practices ensure their virtual visits provide the same patient experience as in-office visits, we’ve developed the e-learning series titled “Telehealth – Processes to maintain a great patient experience.” Two modules are available:
- A module for clinicians focused on patient-centered care and communication tips (15 minutes)
- A module for office managers or leads with tips to ensure telehealth appointments are successful (15 minutes)
For these and other on-demand resources, CME credits are available for physicians and CEU’s are available for medical assistants.
For more information, email PatientExperience@bcbsm.com.
Reminder: How billing works for care authorized by VA at non-VA facilities for FEP members
Veteran Administration benefits are coordinated with Blue Cross and Blue Shield Federal Employee Program® benefits.
When a VA facility authorizes care by a non-VA health care provider, the VA facility is considered the billing provider, and the non-VA provider is considered the performing provider. The VA, as the billing provider, is responsible for filing the member’s claim for the FEP® benefit to the local plan. The non-VA provider is reimbursed by the VA facility.
Starting April 1, additional preferred product required for Soliris, Ultomiris for most commercial members
For dates of service on or after April 1, 2024, step therapy requirements will change for Soliris® (eculizumab), HCPCS code J1300, and Ultomiris® (ravulizumab), HCPCS code J1303.
Preferred products for Soliris and Ultomiris |
Before April 1, 2024 |
On or after April 1, 2024 |
Members must try and fail:
|
Members must try and fail both:
- Rystiggo®
- Either Vyvgart or Vyvgart Hytrulo
|
This change affects Blue Cross Shield of Michigan commercial members and Blue Care Network commercial members.
By April 1, we’ll update the Blue Cross and BCN utilization management medical drug list to reflect the new preferred drugs.
The drugs discussed above continue to require prior authorization through the NovoLogix® online tool.
Some Blue Cross commercial groups aren’t subject to these requirements
For Blue Cross commercial groups, this prior authorization requirement applies only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list.
Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program.
Additional information
For more information about medical benefit drugs, see the following pages on ereferrals.bcbsm.com:
Prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
We’ve changed how we manage Entyvio SC, Omvoh SC
On March 1, 2024, Blue Cross Blue Shield of Michigan and Blue Care Network changed how we manage the following drugs for our Medicare Plus Blue℠ and BCN Advantage℠ members:
- Entyvio® SC (vedolizumab), HCPCS code J3590
- Omvoh™ SC (mirikizumab-mrkz), HCPCS code J3590
Note: This change doesn’t affect Entyvio IV, HCPCS code J3380, or Omvoh IV, HCPCS code J3590, which will continue to be managed as part of members’ Part B medical benefits. These drugs continue to require prior authorization through the NovoLogix® web tool.
What changed on March 1
On March 1, Medicare Plus Blue and BCN Advantage members who previously received Entyvio SC or Omvoh SC under the Part B medical benefit were required to continue their treatments under their Part D pharmacy benefits.
We made this change because these therapies can be safely and conveniently self-administered in the home; the Centers for Medicare & Medicaid Services, or CMS, has added these drugs to the Self-Administered Drug Exclusion List: (SAD List).**
As a result:
- These drugs are no longer covered when administered by a doctor or other health care professional under the Part B medical benefit.
- Entyvio SC and Omvoh SC aren’t included in our Medicare Advantage Part D formularies, but providers can request prior authorization for them as exceptions. (See the “How to submit prior authorization requests for Entyvio SC and Omvoh SC” section below.)
- Your patients can obtain these medications at pharmacies that dispense specialty drugs. They can also obtain these drugs from AllianceRx Walgreens Pharmacy through mail order or pickup at a Walgreens retail pharmacy.
- For members who don’t have Part D pharmacy benefits through Blue Cross or BCN, providers need to work with the pharmacy vendor that provides each member’s Part D coverage.
How to submit prior authorization requests for Entyvio SC and Omvoh SC
For members who have Part D pharmacy benefits through Medicare Plus Blue or BCN Advantage, providers need to submit prior authorization requests for Entyvio SC and Omvoh SC as follows:
List of requirements
For a full list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue and BCN Advantage members.
Authorization isn't a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Additional cardiology codes will require prior authorization for commercial members, starting May 11
For dates of service on or after May 11, 2024, these additional cardiology codes will require prior authorization by Carelon Medical Benefits Management for Blue Cross Blue Shield of Michigan and Blue Care Network commercial members:
Starting May 11, Carelon will use the Blue Cross and BCN medical policy titled Leadless Cardiac Pacemakers as the criteria for making determinations on prior authorization requests. To access this policy, open the Medical Policy Router Search page on bcbsm.com, enter the name of the policy in the Policy/Topic Keyword field and press Enter.
You’ll be able to access Carelon’s clinical criteria for these procedures, when available, on the Current Cardiology Guidelines** page of the Carelon website.
Additional information
By May 11, we’ll update this document to include the previously mentioned codes.
For more resources related to the cardiology procedures that require prior authorization, refer to these webpages at ereferrals.bcbsm.com:
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage prior authorizations for select services.
Webinars for physicians, coders focus on risk adjustment, coding
Beginning in April 2024, we’ll offer webinars about documentation and coding of common challenging diagnoses. These live, lunchtime educational sessions will also include an opportunity to ask questions.
Below is our schedule and the tentative topics for the sessions. All sessions start at noon Eastern time and generally last for 30 minutes. Register for the session that best works with your schedule on the provider training website.
Session date |
Topic |
April 17 |
HCC and Risk Adjustment Updates |
May 22 |
Medical Record Documentation and MEAT |
June 26 |
Orthopedic and Sports Medicine Coding Tips |
July 10 |
Diabetes and Weight Management Coding Tips |
Aug. 21 |
Cardiovascular Disease and Vascular Surgery Coding Tips |
Sept. 18 |
Neurosurgery, Dementia and Cognitive Impairment Coding Tips |
Oct. 2 |
ICD-10-CM Updates |
Nov. 13 |
Oncology Coding Tips |
Dec. 11 |
CPT Updates 2025 |
Provider training website access
Provider portal users with an Availity® Essentials account can access the provider training website by logging in to availity.com,** clicking on Payer Space in the top menu bar and then clicking on the BCBSM and BCN logo. Then click on the Applications tab, scroll down to the Provider Training Site tile and click on it.
You can also directly access the training website if you don’t have a provider portal account: Provider training website.
After logging in to the provider training website, look in Event Calendar to sign up for your desired session. You can also quickly search for all the sessions with the keyword “lunchtime" and then look under the results for Events.

Questions?
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
|


