|
December 2020

After moving to Availity in 2021, many of our current online tools will still be available
 Blue Cross Blue Shield of Michigan and Blue Care Network will move our secure provider website to the Availity® Provider Portal in 2021. We’re going to focus on different aspects of this transition through a series of articles in this publication over the next year. Blue Cross Blue Shield of Michigan and Blue Care Network will move our secure provider website to the Availity® Provider Portal in 2021. We’re going to focus on different aspects of this transition through a series of articles in this publication over the next year.
Here are the articles we’ve already published, in case you missed them:
This month, we’re focusing on the tools that won’t change with the move to Availity.
What’s not changing
Change can be exciting, but it can be difficult, too. While there will be new things to learn in the move to Availity, many of the tools that you’re familiar with will remain. The only change will be how to find those tools.
Once Blue Cross and BCN move to the Availity Provider Portal next year, you’ll log in to Availity rather than logging in to the Blue Cross and BCN Provider Secured Services website at bcbsm.com. As we get closer to the 2021 launch date, we’ll provide you with training and specific step-by-step instructions to make it easy for you to find what you need.
Here’s a list of tools you’ll still be able to find and use once we move to the Availity Provider Portal. Please note that some of these tools may only be available to certain providers based on their access role:
- e-referral (for managing referrals and authorization requests)
- BCBSM Pharmacy Benefit — Medication Prior Authorization
- BCBSM, BCN and Medicare Advantage PPO Medical Benefit — Medication Prior Authorization/NovoLogix®
- Health e-Blue℠ (patient data registry and treatment opportunities for primary care physicians and groups)
- Benefit Explainer (benefit details for Blue Cross commercial members with coverage from employer groups located within Michigan)
- Provider Enrollment and Change Self-Service
- BCBSM Behavioral Health Preservice Review
- Clear Claim Connection™ (for Michigan providers to view claim edits)
- Internet Claims Tool
- BCN Negative Balance Reports
- BCBSM Qualification Form
Many of the other resources you use will also continue to be available, including: Entire Fee schedules and Fee Changes, Provider Publications and Resources, Provider Manuals, the Medical Policy and Pre-Cert/Pre-Auth Router and Find a Doctor.
In future issues of The Record, we’ll provide information on the new and updated features you’ll find in Availity, how to register for Availity and everything you’ll need to know to transition to the Availity Provider Portal for your Blue Cross and BCN patient needs.
Reminder: Billing for COVID-19 testing
With the increase in COVID-19 cases that we’re seeing across Michigan and the United States, we want to remind our health care providers about Blue Cross Blue Shield of Michigan and Blue Care Network’s policy when it comes to COVID-19 testing.
Blue Cross and BCN will cover the cost of COVID-19 testing for members that:
- Is ordered by a qualified health professional who determines testing is medically appropriate, using judgment in accordance with accepted standards of current medical practice; and
- Has met the necessary regulatory approval through the FDA or falls within one of the other categories of tests required to be covered by the Families First or CARES acts.
The test orders must show medical necessity. The only exception is for patients with Medicare Advantage coverage who are allowed one COVID-19 test without an order from a health professional in accordance with the Centers of Medicare & Medicaid Services policy.
Blue Cross and BCN cover pre-operative COVID-19 testing for procedures conducted in hospital operating rooms and ambulatory surgical facilities. Aerosol-generating procedures are also appropriate for pre-operative COVID-19 testing, regardless of the location performed, such as oral surgery in an office setting.
It’s important to note that Blue Cross and BCN policy does not cover workplace or screening tests, such as testing to:
- Participate in sports
- Return to work or school
- Qualify for admission to armed services, residential facilities, etc.
- Engage in research
- Accommodate requests for routine testing due to general concerns or a desire to get tested prior to family gatherings, vacations, etc.
If a patient wants to get testing that their health plan won’t cover, you can direct them to Michigan.gov/coronavirus** to find a site that offers free COVID-19 testing.
For more information, see the COVID-19 patient testing recommendations document. It’s available on our public website at bcbsm.com/coronavirus and by logging in as a provider at bcbsm.com and clicking on Coronavirus (COVID-19).
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
CareCentrix® to manage prior authorizations for home health care for Medicare Advantage members
Blue Cross Blue Shield of Michigan and Blue Care Network have contracted with CareCentrix to manage authorization of home health care for Medicare Advantage members.
For dates of service on or after March 1, 2021, providers will need to request prior authorization from CareCentrix for Medicare Plus Blue℠ and BCN Advantage℠ members for home health care.
CareCentrix will authorize and support the coordination of home health care services, such as skilled nursing and physical, occupational and speech therapies.
Submitting prior authorization requests
Primary care providers, acute care providers, post-acute care providers, and home health care agencies will be able to submit requests online through the CareCentrix portal, by phone, by fax and through AllScripts®.
How does this benefit your patients?
This home health care program is designed to:
- Reduce the length of stay in inpatient facilities.
- Lower the chance of hospital readmission.
- Assist with the transition from hospital to home.
- Provide a home-based center of care.
Claims and appeals
Home health agencies will continue to submit claims, claims questions and appeals to Blue Cross Blue Shield of Michigan.
If providers do not obtain authorization for home health care from CareCentrix, claims may be denied.
Next steps
In future newsletter articles and web-DENIS messages, we’ll provide more information about CareCentrix and this change, including:
- Detailed information about portal access and the prior authorization process
- How to sign up for training webinars
- How to access resources and support
CMS approves Next Generation Sequencing for certain cancer patients
The Centers for Medicare & Medicaid Services has determined that Next Generation Sequencing is covered as a diagnostic laboratory test for patients with germline (inherited) ovarian or breast cancer when:
- It’s performed in a Clinical Laboratory Improvement Amendments, or CLIA, certified laboratory.
- It’s ordered by a treating physician.
- Specific requirements are met.
The CMS decision is retroactive to Jan. 27, 2020, and affects all Medicare Advantage plans, including Medicare Plus Blue℠ and BCN Advantage℠. The implementation date for claims processing was Nov. 13, 2020.
Next Generation Sequencing is a technique that can measure one or more genetic variations as a laboratory diagnostic test, such as when used in conjunction with an in vitro diagnostic test.
The decision memo** about this topic is available on the CMS website.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
State of Michigan group offers high-deductible health plan with an HSA to active employees
State of Michigan active employees now have the option to enroll in Blue Cross Blue Shield of Michigan’s Simply Blue℠ HSA commercial health plan. Michigan State Police Troopers Associated employees are excluded from this offering.
Key things to know
- The State of Michigan group number is 007000562.
- The plan’s effective date is Jan.1, 2021, and is referred to as the State High Deductible Health Plan with an HSA. The plan is based on Blue Cross’ Simply Blue HSA certificate.
- Simply Blue HSA is a high-deductible plan that includes a health savings account administered through HealthEquity®, an aggregate deductible and an embedded out-of-pocket maximum. HealthEquity is an independent company that supports Blue Cross by providing health care spending account administration services.
- Enrollees will have access to Blue Cross’ broad PPO (commercial) network.
- New Directions® Behavioral Health will continue to manage behavioral health and substance use disorder benefits on behalf of Blue Cross for State of Michigan enrollees. New Directions Behavioral Health is an independent company that provides behavioral health services for most Blue Cross members.
- OPTUMRx® is the prescription drug carrier under the plan. OptumRx is an independent company supporting Blue Cross by providing pharmacy services.
What you need to do, starting Jan. 1
- Check your patient’s Blue Cross member ID cards and web-DENIS for eligibility and benefit information. New member ID cards will be issued throughout the month of December.
- Submit all medical and behavioral health claims to Blue Cross.
Meijer to move to exclusive provider organization network arrangement and offer an HPN option
Starting Jan. 1, 2021, all plans for Meijer members (group number 72625) will move to an exclusive provider organization, or EPO, network arrangement. In addition, Meijer is offering a High-Performance Network, or HPN, option to its members. We’ve told you about Blue Cross’ HPN in recent articles that appeared in the October and November issues of The Record.
In these types of plans, members are responsible for obtaining services from in-network providers and services obtained out-of-network are not a benefit (with some exceptions).
In particular, we wanted to call your attention to services provided by AIM Specialty Health. AIM provides services like high-tech radiology, cardiology and in-lab sleep studies. Prior authorizations are required for any services provided by AIM. You can contact AIM for prior authorization through their website at AIMSpecialtyHealth.com** or by calling AIM’s call center at 1-800-728-8008.
For more information about the Meijer group changes, effective Jan. 1, see the “Group benefit changes” section of the December billing chart.
You’ll know if your patient has a Meijer Blue HPN plan by their Blue Cross ID card or in web-DENIS when you check eligibility. An image of a sample card is below.
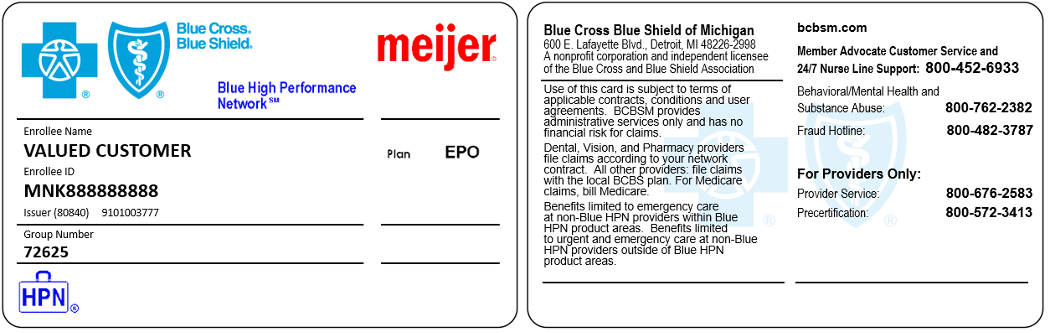
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Billing chart: Blues highlight medical, benefit policy changes
You’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart.
This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures.
You will also see that descriptions for the codes are no longer included. This is a result of recent negotiations with the AMA on use of the codes.
We will publish information about new BCBS groups or changes to group benefits under the Group Benefit Changes heading.
For more detailed descriptions of the BCBSM policies for these procedures, please check under the Medical/Payment Policy tab in Explainer on web-DENIS. To access this online information:
- Log in to web-DENIS.
- Click on BCBSM Provider Publications & Resources.
- Click on Benefit Policy for a Code.
- Click on Topic.
- Under Topic Criteria, click on the drop-down arrow next to Choose Identifier Type and then click on HCPCS Code.
- Enter the procedure code.
- Click on Finish.
- Click on Search.
| Code* |
BCBSM changes to:
Basic Benefit and Medical Policy, Group
Variations Payment Policy, Guidelines
|
| UPDATES TO PAYABLE PROCEDURES |
0017U, 81219, 81270, 81402, 81403, 81450 |
Basic benefit and medical policy
Genetic Testing ̶ JAK2, MPL and CALR Testing for Myeloproliferative Neoplasms policy
The medical policy statement has been updated for the Genetic Testing ̶ JAK2, MPL and CALR Testing for Myeloproliferative Neoplasms policy.
Medical policy statement
The safety and effectiveness of JAK2 testing have been established. It may be considered a useful diagnostic option for patients presenting with clinical, laboratory or pathologic findings suggesting polycythemia vera, essential thrombocythemia or primary myelofibrosis.
The safety and effectiveness of MPL and CALR testing have been established. They may be considered useful diagnostic options for patients presenting with clinical, laboratory or pathologic findings suggesting essential thrombocythemia or primary myelofibrosis.
The use of a targeted genomic panel for hematolymphoid neoplasms may be considered appropriate for the diagnosis and for the selection of the therapy for a myeloproliferative disorder or myelodysplastic syndrome.
The peer-reviewed medical literature hasn’t yet demonstrated the clinical utility for JAK2, MPL and CALR testing in other circumstances. Therefore, these services are considered experimental in all other situations including, but not limited to, the following:
- Diagnosis of nonclassic forms of myeloproliferative neoplasms, or MPNs
- Molecular phenotyping of patients with MPNs
Inclusions:
JAK2 testing as a diagnostic option for patients presenting with clinical, laboratory or pathologic findings suggesting polycythemia vera, essential thrombocythemia or primary myelofibrosis.
Based on World Health Organization criteria, in the case of suspected polycythemia vera, documentation of serum erythropoietin, or EPO, level below the reference range for normal is recommended prior to JAK2 testing. (Refer to the medical policy for the policy guidelines.)
MPL and CALR testing as diagnostic options for patients presenting with clinical, laboratory or pathologic findings suggesting essential thrombocythemia or primary myelofibrosis.
The use of a targeted genomic panel for hematolymphoid neoplasms may be considered appropriate for the diagnosis and for selection of the therapy for a myeloproliferative disorder or myelodysplastic syndrome, as well as targeted therapy.
Exclusions:
JAK2, MPL and CALR testing in other circumstances including, but not limited to, the following:
- Diagnosis of nonclassic forms of myeloproliferative neoplasms, or MPNs
- Molecular phenotyping of patients with MPNs
This policy is effective Nov. 1, 2020. |
38204, 38205, 38206, 38207, 38208, 38209, 38210, 38211, 38212, 38213, 38214, 38215, 38230, 38232, 38240, 38241, 38242, 38243, 81265, 81266, 81267, 81268, 81370, 81371, 81372, 81373, 81374, 81375, 81376, 81377, 81378, 81379, 81380, 81381, 81382, 81383, 86812, 86813, 86816, 86817, 86821, S2140, S2142, S2150 |
Basic benefit and medical policy
BMT-HCT for Hodgkin Lymphoma policy
The safety and effectiveness of autologous or myeloablative allogeneic hematopoietic cell transplantation, or HCT, and reduced-intensity allogeneic HCT have been established. They can be useful therapeutic options for patients with primary refractory or relapsed Hodgkin lymphoma who meet patient selection criteria.
Other uses for HCT are experimental.
The exclusions have been updated to include tandem autologous HCT, effective Jan. 1, 2021.
Inclusions:
Autologous HCT:
- Patients with primary refractory HL
- Patients with relapsed HL
Allogeneic HCT, using either myeloablative or reduced intensity conditioning HCT:
- Patients with primary refractory HL
- Patients with relapsed HL
Exclusions:
- A second autologous cell transplant for relapsed lymphoma after a prior autologous HCT
- Other uses of HCT in patients with HL including, but not limited to, initial therapy for newly diagnosed disease to consolidate a first complete remission
- Tandem autologous HCT
|
| POLICY CLARIFICATIONS |
J0584 |
Basic benefit and medical policy
Crysvita (burosumab-twz)
Crysvita (burosumab-twz) is payable for the following updated indication:
- The treatment of tumor-induced osteomalacia in patients 2 years and older
This drug isn’t a benefit for URMBT. |
J3490
J3590 |
Basic benefit and medical policy
Olinvyk (oliceridine)
Effective Aug. 7, 2020, Olinvyk (oliceridine) is covered for the following FDA-approved indications:
Olinvyk (oliceridine) is an opioid agonist indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate.
Limitations of use
Because of the risks of addiction, abuse and misuse with opioids, even at recommended doses, reserve Olinvyk for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or opioid combination products):
- Haven’t been tolerated or aren’t expected to be tolerated
- Haven’t provided adequate analgesia or aren’t expected to provide adequate analgesia
The cumulative total daily dose shouldn’t exceed 27 mg.
Dosage and administration
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals.
- Individualize dosing based on the severity of pain, patient response, prior analgesic experience and risk factors for addiction, abuse and misuse.
- Initiate treatment with a 1.5 mg dose.
- For patient-controlled analgesia, or PCA, recommended demand dose is 0.35 mg, with a six-minute lock-out. A demand dose of 0.5 mg may be considered.
- Supplemental doses of 0.75 mg can be administered, beginning one hour after the initial dose and hourly thereafter, as needed.
- Don’t stop Olinvyk in a physically dependent patient.
Dosage forms and strengths
- 1 mg/mL and 2 mg/2 ml (1 mg/mL) in single-dose vials
- 30 mg/30 mL (1 mg/mL) in single-patient-use vial, for PCA use only
This drug isn’t a benefit for URMBT. |
Established
20932, 20933, 20934, 27415, 27416, 28446, 29866, 29867, 29879
Experimental
27899, 29999 |
Basic benefit and medical policy
Autografts and Allografts in the Treatment of focal Articular Cartilage Lesions policy
The medical policy statement has been updated for the Autografts and Allografts in the Treatment of focal Articular Cartilage Lesions policy. This policy is effective Nov. 1, 2020.
Medical policy statement
Microfracture technique
The safety and effectiveness of microfracture surgery in joints (e.g., knee, hip, shoulder) for the treatment of osteochondritis dissecans, or OCD, has been established in patients where OCD is proven.
Osteochondral allografting
The safety and effectiveness of osteochondral allografting to repair large, full-thickness chondral defect of the knee or talus caused by acute or repetitive trauma have been established. It’s a useful therapeutic option for selected patients.
Osteochondral allografting for all other joints is experimental. It hasn’t been shown to improve patient outcomes better than conventional treatment.
Osteochondral autografting
The safety and effectiveness of osteochondral autografting, using one or more cores of osteochondral tissue, has been established for the treatment of symptomatic full-thickness cartilage defects of the knee or talus caused by acute or repetitive trauma in patients who have had an inadequate response to a prior surgical procedure, when the inclusionary criteria are met.
Osteochondral autografting for all other joints and any indications other than those listed above is considered experimental.
Treatment of focal articular cartilage lesions with autologous or allogeneic minced cartilage is considered experimental.
Treatment of focal articular cartilage lesions with decellularized osteochondral allograft plugs (e.g., Chondrofix, TrueFit) is considered experimental.
Treatment of focal articular cartilage lesions with reduced osteochondral allograft discs (e.g., ProChondrix, Cartiform, DeNovo Engineered Tissue, BioCartilage®) is considered experimental. |
69710, 69711, 69714, 69715, 69717, 69718, L8625, L8690, L8691, L8692, L8693, L8694 |
Basic benefit and medical policy
Unilateral or bilateral fully or partially implanted bone-conduction (bone-anchored) hearing aids
The safety and effectiveness of FDA-approved unilateral or bilateral fully or partially implanted bone-conduction (bone-anchored) hearing aids have been established. They may be considered a useful therapeutic option when indicated.
The use of a Baha® Softband may be considered established in children 5 years of age and younger meeting criteria for BAHA treatment, but who are determined to have inadequate skeletal maturity to sustain osteointegration of the Baha device.
Inclusionary criteria have been updated, effective Nov. 1, 2020.
Inclusions:
FDA-approved devices when used according to approved indications and guidelines.
Conductive hearing loss:
FDA-approved unilateral or bilateral fully or partially implantable bone-conduction (bone-anchored) hearing aids may be necessary as an alternative to an air-conduction hearing aid in patients with conductive or mixed hearing loss 5 years of age and older (Baha 4, Baha 5, Baha 5 SuperPower, Baha Cordele II, PontoTM Bone Anchored Hearing System, Ponto 4 and Otomag® Bone Conduction [OBC] devices) or 12 years of age and older (OSIA II system) who also meet one of the following criteria:
- Congenital or surgically induced malformations (e.g., atresia) of the external ear canal or middle ear
- Chronic external otitis or otitis media
- Tumors of the external canal or tympanic cavity
- Chronic dermatitis of the external canal prohibiting the usage of an air conduction hearing aid
And meet the following audiologic criteria:
- A pure-tone average bone-conduction threshold measured at 0.5, 1, 2, and 3 kHz or better than or equal to one of the following:
- 45 dB (OBC and BP100, Baha 4 and Baha 5, Ponto, Ponto 3, Ponto Pro, Ponto Plus, and Ponto 4 devices)
- 55 dB (Intenso, OSIA II, Ponto 3 power, Ponto Pro Power and Ponto Plus Power devices)
- 65 dB (Cordele II, Baha 5 SuperPower, Ponto 3 SuperPower devices).
For bilateral implantation, patients should meet the above audiologic criteria in both ears and have symmetrically conductive or mixed hearing loss as defined by a difference between left and right side bone-conduction threshold of less than 10 dB on average measured at 0.5, 1, 2, and 3 kHz (4 kHz for OBC, Ponto Bone Anchored Hearing System, Ponto 3, Ponto 3 Power, Ponto 3 SuperPower, Ponto 4 and Ponto Pro devices), or less than 15 dB at individual frequencies.
Sensorineural hearing loss:
A unilateral implantable bone-conduction (bone-anchored) hearing aid may be considered medically necessary as an alternative to an air-conduction contralateral routing of signal hearing aid in patients 5 years of age and older (Baha 4, Baha 5, Baha 5 SuperPower, Baha Cordele II, OBC, Ponto 3, Ponto 3 Power and Ponto 3 SuperPower and Ponto Bone Anchored Hearing devices) or 12 years of age and older (OSIA II system) with single-sided sensorineural deafness and normal hearing in the other ear. The pure-tone average air-conduction threshold of the normal ear should be better than 20 dB measured at 0.5, 1, 2, and 3 kHz.
Note: The Audiant® bone conductor is a bone-conduction hearing device. While this product is no longer actively marketed, patients with existing Audiant devices may require replacement, removal or repair.
In patients being considered for implantable bone-conduction (bone-anchored) hearing aid(s), skull bone quality and thickness should be assessed for adequacy to ensure implant stability. Additionally, patients (or caregivers) must be able to perform proper hygiene to prevent infection and ensure the stability of the implants and percutaneous abutments.
Exclusions:
- Other uses of implantable bone-conduction (bone-anchored) hearing aids, including use in patients with bilateral sensorineural hearing loss, are considered experimental.
- Non-FDA-approved devices or indications.
|
69930, 92601, 92602, 92603, 92604, 92605 92606, 92607, 92608, 92609, 92618, L7510, L8614, L8615, L8616, L8617, L8618, L8619, L8621, L8622, L8623, L8624, L8625, L8627, L8628, L8629 |
Basic benefit and medical policy
Cochlear Implant policy
The safety and effectiveness of U.S. Food and Drug Administration‑approved bilateral and unilateral cochlear implants and associated hybrid cochlear implant devices have been established. The implants may be considered useful therapeutic options when indicated.
Inclusionary criteria have been updated, effective Nov. 1, 2020.
Inclusions:
Bilateral or unilateral cochlear implantation is considered an established, safe and effective therapy if all the following criteria are met:
- FDA-approved cochlear implant
- 9 months of age or older
- Bilateral severe to profound pre- or post-lingual (sensorineural) hearing loss
- Defined as a hearing threshold of pure-tone average of 70 dB hearing loss or greater at 500, 1000, 2000 Hz
- Limited or no benefit from hearing aids
Unilateral cochlear implantation is considered established, safe and effective therapy in single-sided deafness, or SSD,a,b when all following are met:
- FDA-approved cochlear implant
- 5 years of age or older
- Profound sensorineural hearing loss in one ear and normal hearing or mild sensorineural hearing loss in the other ear
- Profound hearing loss is defined as having a pure-tone average of 90dB hearing loss or greater at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz. Normal hearing is defined as having a PTA of up to 15 dB HL at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz. Mild hearing loss is defined as having a PTA of up to 30 dB HL at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz. Mild to moderately severe hearing loss is defined as having a PTA ranging from 31 to up to 55 dB HL at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz.
aIndividuals with single sided deafness, or SSD, or asymmetrical hearing loss, or AHL, must obtain limited benefit from an appropriately fitted unilateral hearing aid in the ear to be implanted. For individuals ages 18 and older, limited benefit from unilateral amplification is defined by test scores of 5% correct or less on monosyllabic consonant-nucleus-consonant, or CNC, words in quiet when tested in the ear to be implanted alone. For individuals between ages 5 and 18, insufficient functional access to sound in the ear to be implanted.
bAHL is defined as a profound sensorineural hearing loss in one ear and mild to moderately severe sensorineural hearing loss in the other ear, with a difference of at least 15 dB in pure tone averages, or PTAs, between ears.
Replacement of internal or external components in a small subset of members may be considered established when all the following are met:
- There’s an inadequate response to existing components to the point of one of the following:
- Interfering with the individual’s activities of daily living.
- The components are no longer functional and can’t be repaired.
- Copies of original medical records must be submitted, either hard copy or electronically, to support medical necessity.
Cochlear implant with a hybrid device that includes the hearing aid integrated into the external sound processor of the cochlear implant (e.g., the Nucleus® Hybrid L24 Cochlear Implant System) may be considered established for patients 18 years and older who meet all of following criteria:
- Bilateral severe-to-profound high frequency sensorineural hearing loss with residual low-frequency hearing sensitivity
- Receive limited benefit from appropriately fit bilateral hearing aids
- Have the following hearing thresholds (must meet all):
- Low frequency hearing thresholds no poorer than 60 dB hearing level up to and including 500 Hz (averaged over 125, 250, and 500 Hz) in the ear selected for implantation
- Severe to profound mid-to-high frequency hearing loss (threshold average of 2000, 3000, and 4000 Hz ≥75 dB hearing level) in the ear to be implanted
- Moderately severe to profound mid-to-high frequency hearing loss (threshold average of 2000, 3000, and 4000 Hz ≤ 60 dB hearing level) in the contralateral ear
- Aided consonant-nucleus-consonant word recognition score from 10% to 60% in the ear to be implanted in the preoperative aided condition and in the contralateral ear will be equal to or better than that of the ear to be implanted but not more than 80% correct.
In certain situations, implantation may be considered before age 9 months. One scenario: Post-meningitis when cochlear ossification may preclude implantation. Another scenario is in cases with a strong family history, because establishing a precise diagnosis is less uncertain.
Contraindications to cochlear implantation may include deafness due to lesions of the eighth cranial (acoustic) nerve, central auditory pathway or brainstem; active or chronic infections of the external or middle ear; and mastoid cavity or tympanic membrane perforation. Cochlear ossification may prevent electrode insertion, and the absence of cochlear development as demonstrated on computed tomography scans remains an absolute contraindication.
Exclusions:
- Upgrades of an existing, functioning external system to achieve aesthetic improvement, such as smaller profile components or a switch from a body-worn, external sound processor to a behind-the-ear model
- Replacement of internal or external components solely for the purpose of upgrading to a system with advanced technology or to a next-generation device
- Non-FDA approved devices or indications
|
Telemedicine – Synchronous
99441, 99442, 99443, 99421, 99422, 99423, any CPT code that is appropriate for both the encounter and provider scope.
Experimental
H0031, H0032, H2014, H2019, 0362T, 97151, 97152, 97153, 97154, 97158
Telemedicine asynchronous (store and forward)
99446, 99447, 99448, 99449, 99451
99452, G2010
Behavioral health codes
Established only the following codes:
96130 and 96156
Behavioral health codes
Other codes: (experimental, not medically necessary, etc.)
All CPT codes related to behavioral health care, other than those listed under “Established” |
Basic benefit and medical policy
Telemedicine Services policy
The criteria have been updated for the Telemedicine Services policy. Historically, telemedicine, a subset of telehealth, has been defined as the use of telecommunications technology for real-time, medical diagnostic and therapeutic purposes when distance separates the patient and health care provider.
In June 2020, influenced by the COVID-19 pandemic, the State of Michigan expanded the definition of telemedicine to include store and forward (asynchronous) as well as real-time (synchronous) interactions.
With this expanded definition, telemedicine now includes the synchronous and asynchronous delivery of care between a patient and provider, or between provider and provider. Telemedicine may substitute for a face-to-face, hands-on encounter between a patient and the health care provider when using the appropriate technology.
The safety and effectiveness of telemedicine (synchronous or asynchronous care) have been established. It may be considered a useful diagnostic and therapeutic option when indicated.
Inclusions:
Synchronous (real-time encounter)
- The provider must be licensed, registered or otherwise authorized to perform service in their health care profession in the state where the patient is located. Services must fall within their scope of practice.
- Telemedicine delivered services are available to all clinicians; however, this may not be the preferred method of delivery in certain clinical scenarios, for example chronic suicidal ideation or unstable angina. A hosted visit** or a face-to-face visit may be necessary due to the complexity of the clinical situation. The telemedicine provider may provide the face-to-face encounter.
- Telemedicine delivered services for ongoing treatment of a condition that is chronic or is expected to take more than five sessions before the condition resolves or stabilizes may require a hosted visit** or a face-to-face visit. The telemedicine provider may provide the face-to-face encounter.
- The service must be conducted over a secured channel.**
- The delivery of the service can be either audio only (telephone) or audio/video (a secured computer-based system).
**See Policy Guidelines
Online visit
- An audio-visual online communication
- The patient initiates the medical or behavioral health encounter
- The provider must be licensed, registered or otherwise authorized to perform service in their health care profession in the state where the patient is located.
- A low-complexity, straightforward decision-making encounter that addresses urgent but not emergent clinical conditions
- A single encounter where a follow-up encounter isn’t anticipated
- Services must fall within the provider’s scope of practice.
Asynchronous (store and forward encounter)
- The provider must be licensed, registered or otherwise authorized to perform service in their health care profession in the state where the patient is located. Services must fall within their scope of practice.
- The patient data (pre-recorded videos, digital images such as X-rays or photos, test results or any other information necessary for the evaluation) must be transmitted over a secured channel.**
- Behavioral health services allowed as asynchronous care are limited. (See the Billing Guidance and Code sections.)
**See Policy Guidelines.
Exclusions – synchronous and asynchronous:
- Request for medication refills
- Reporting of normal test results
- Provision of educational materials
- Scheduling of appointments and other health care related issues
- Registration or updating billing information
- Reminders for health care related issues
- Referrals to other providers
- An online or telemedicine visit resulting in an office visit, urgent care or emergency care encounter on the same day for the same condition
- An online visit for the same condition of an online visit within the previous seven days
- An online or telemedicine visit occurring during the post-operative period
Applied behavioral analysis for the treatment of autism spectrum disorder is considered experimental when delivered by telemedicine (synchronous and asynchronous care).**
**Exceptions:
- Parent/guardian/caregiver adaptive behavior treatment training (97156, 97157, S5111, S5108) may be performed as a telemedicine service.
- Program modification of ABA therapy (97155) may be used as a combination of face-to-face and telemedicine services up to 50% of the time – as long as a technician is present face to face.
Note: Please refer to the medical policy for the Policy Guidelines.
The guidelines:
- Define who is an eligible provider
- Explain how we cover applied behavior analysis therapy for autism spectrum disorder
- Outline expectations for secure communication with patients
- Provide information regarding appropriate billing (e.g., an originating site is no longer required)
This policy is effective Nov. 1, 2020. |
J3490
J3590 |
Basic benefit and medical policy
Byfavo (reminazolam)
Effective July 2, 2020, Byfavo (reminazolam) is covered for the following FDA-approved indications:
Byfavo (remimazolam) for injection is a benzodiazepine indicated for the induction and maintenance of procedural sedation in adults undergoing procedures lasting 30 minutes or less.
Dosage and administration
Individualize and titrate BYFAVO dosing to desired clinical effect.
Adult patients:
- Administer an initial dose intravenously as a 5 mg push injection over a one-minute time period.
- If necessary, administer supplemental doses of 2.5 mg intravenously over a 15-second time period. At least two minutes must elapse prior to the administration of any supplemental dose.
ASA-PS III-IV patients (at the discretion of the physician):
- Based on the general condition of the patient, administer 2.5 mg to 5 mg over a one-minute time period.
- If necessary, administer supplemental doses of 1.25 mg to 2.5 mg intravenously over a 15-second time period. At least two minutes must elapse prior to the administration of any supplemental dose.
Dosage forms and strengths
Each glass, single-patient-use vial contains 20 mg BYFAVO (remimazolam) lyophilized powder for reconstitution, equivalent to 27.2 mg remimazolam besylate.
This drug isn’t a benefit for URMBT. |
J7298 |
Basic benefit and medical policy
Mirena (levonorgestrel-releasing intrauterine system)
Effective Aug. 20, 2020, Mirena (levonorgestrel-releasing intrauterine system) is covered for the following FDA-approved indications:
- Prevention of pregnancy for up to 6 years
- Treatment of heavy menstrual bleeding for women who choose to use intrauterine contraception as their method of contraception for up to five years.
Dosage and administration
- Initial release rate of levonorgestrel (LNG) is 20 mcg/day; this rate is reduced to about 10 mcg/day after 5 and 9 mcg/day after 6 years.
- To be inserted by a trained health care provider using strict aseptic technique. Follow insertion instructions exactly as described.
Patient should be re-examined and evaluated four to six weeks after insertion; then, yearly or more often if indicated. |
| EXPERIMENTAL PROCEDURES |
43644, 43645 |
Basic benefit and medical policy
Gastric bypass surgery for gastroparesis
Gastric bypass surgery for gastroparesis is experimental. This procedure hasn’t been scientifically demonstrated to be as safe and effective as conventional treatment.
The policy effective date is Nov. 1, 2020. |
64999, C9757 |
Basic benefit and medical policy
Annular closure devices
The use of annular closure devices (e.g., Xclose®, Barricaid®, DART system, Inclose™) is experimental. It hasn’t been clinically demonstrated to be as safe and effective as conventional treatment.
This policy is effective Nov. 1, 2020. |
| GROUP BENEFIT CHANGES |
AJM Packaging |
AJM Packaging, group number 71395, is offering the plans below, effective Jan. 1, 2021.
Group number: 71395
Alpha prefixes: PPO (PYJ), Medicare PPO (PZJ)
Platform: NASCO Hybrid
Plans offered:
PPO medical/surgical
Dental
Vision
Prescription drug |
Meijer |
Effective Jan. 1, 2021, all Meijer plans are moving to an Exclusive Provider Organization, or EPO. In addition, Meijer has implemented the High-Performance Network, or HPN. The HPN is offered as an additional option to existing plans and not as a full replacement. For more details, see the article in this issue of The Record.
Group number: 72625
Alpha prefixes:
EPO (MJE)
EPO WI Select Network (OLI)
EPO High Performance Network (MNK)
Platform: NASCO
Plans offered: All plans are EPO and include hearing:
EPO — Advantages Health with HSA
EPO — Health Select with HRA
HPN — Advantages Health with HSA
HPN — Health Select with HRA
|
Stock X LLC |
Stock X LLC (former group number 71809) is leaving the Rock Central (group number 71544) umbrella and becoming its own group/entity.
Group number: 71822
Alpha prefix: PPO (JXP)
Platform: NASCO
Plans offered:
PPO medical/surgical
Prescription drug
CDH — HSA
Hearing |

Michigan law prohibits ‘surprise billing’
As of Oct. 22, 2020, Michigan state law prohibits surprise billing by nonparticipating professional providers in Michigan for emergency services and some non-emergency services provided on and after this date. Participating providers already agree not to balance bill our members.
“Surprise billing” refers to instances where a member unknowingly receives care from a nonparticipating provider and later receives an unexpected bill for the difference between the insurer’s payment and what the provider charges.
Nonparticipating professional providers in Michigan will no longer be able to balance bill members in the following scenarios outlined in the law:
- Covered emergency services at a participating or nonparticipating health facility
- Covered non-emergency services at a participating health facility when at least one of the following events occurs:
- The patient doesn’t have the ability or opportunity to choose a participating provider; or
- The nonparticipating provider doesn’t provide the required advanced written disclosure notice to the member of the service’s estimated costs and notice of the right to seek care from a participating provider (see Public Act 235)
- A health care service at a participating health facility for a patient who was admitted to the hospital within 72 hours after receiving a health care service in the hospital’s emergency room
For the above scenarios, the law defines the benchmark rate that these nonparticipating providers must now accept as payment in full. The benchmark rate is defined as the greater of the following (excluding any in-network cost share):
- The median amount negotiated by the patient’s carrier for the region and provider specialty, or
- 150% of the Medicare fee-for-service amount listed on the fee schedule for the health care service provided
Members are responsible for any in-network cost-sharing requirements.
If you have questions, contact Provider Inquiry at the appropriate number below:
- Blue Cross Blue Shield of Michigan
- Physicians and other professional providers of care: 1-800-344-8525
- Hospital and facility providers: 1-800-249-5103
- Blue Care Network
- Professional providers: 1-800-344-8525
- Ancillary and facility providers:1-800-249-5103
New clinical editing resource helps you correct billing errors
We’ve posted a new billing tips resource, titled Clinical Edits: What You Need to Do and What Documentation is Needed, in the Provider Secured Services area of bcbsm.com. It’s expected to provide a clearer understanding of the clinical edits you may encounter and how to resolve them.
To find it, follow these steps:
- After logging in to Provider Secured Services, click on BCBSM Provider Publications and Resources.
- Click on Newsletters and Resources and then on Forms.
- Under Clinical Editing Appeal Form, click on Clinical Edits: What You Need to Do and What Documentation is Needed.
Once you open the document, you can use the keyword search function (Ctrl + F) to search for keywords found on your voucher. Here’s a screenshot of a portion of the new resource:
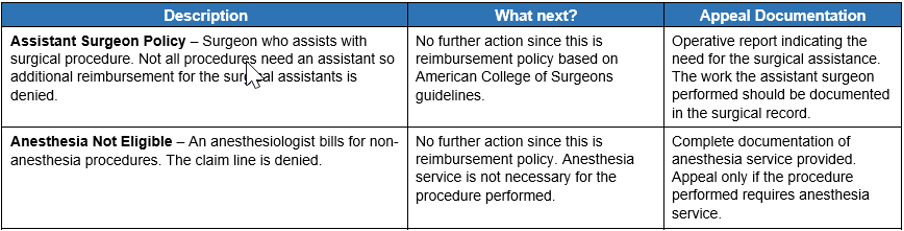
The document provides a description of our clinical editing policies. We follow nationally recognized rules and guidelines from the Centers for Medicare & Medicaid Services, current procedural terminology codes and guidance from professional practitioner associations and societies. Note: The rules aren’t all‑encompassing and are intended to provide additional guidance and understanding of clinical edits.
The document also outlines your options for correcting billing errors. We offer advice on how to proceed to correct a claim or submit an appeal and specify the required appeal documentation.
In addition to Clinical Edits: What You Need to Do and What Documentation is Needed, you’ll find another useful document on the site: Blue Cross Commercial EX codes: Recommendations Regarding Appeal or Resubmission. It offers a description of EX codes and recommendations on whether you should appeal or resubmit.
Keep in mind that there are many online medical billing and coding resources that can help you understand correct coding guidelines. Accurate claim submission and medical record documentation are crucial to correct reimbursement.
As a reminder, clinical editing is an integral part of our payment policy and you’ll need to use the established guidelines for resolving medical and benefit policy questions.
We’re here for you — virtually
We recognize this has been a challenging year. Although we’ve been unable to meet with you in person for the last several months, Blue Cross Blue Shield of Michigan and Blue Care Network’s provider consultants continue to work hard to assist health care providers virtually.
Our consultants continue to be available to help meet your education and training needs, and to help clarify medical policy and contract information. We encourage you to use our self-service tools and Provider Inquiry phone numbers when you need help with claims. When your issue isn’t resolved through these channels, contact your consultant and provide the reference number given to you by the Provider Inquiry representative.
We also offer individualized training as needed, but keep in mind that we offer information on a wide range of topics on our Provider Training and Leaning Opportunities sites.
To learn more about what we offer to all providers, log in to Provider Secured Services and follow these steps:
For Blue Cross
- Click on BCBSM Provider Publications and Resources.
- Clickon BCBSM Newsletters and Resources.
- Click on Provider Training in the left navigation.
For BCN
- Click on BCN Provider Publications and Resources.
- Click on Learning Opportunities.
While we continue to make virtual visits, we’ll consider an in-person visit if your need is urgent. You can request a visit by calling your consultant. The consultant will share our requirements for a safe, in-person meeting.
See our flyer for Blue Cross and BCN contact information plus more details about the role of your provider consultant.
AMC Health remote monitoring program helps Medicare Advantage members who have CHF
Blue Cross Blue Shield of Michigan and Blue Care Network are working with AMC Health to offer remote patient monitoring to eligible members with congestive heart failure. This offering, which went into effect Aug. 1, 2020, is available to BCN Advantage℠ and Medicare Plus Blue℠ members.
The goal of the program is to reduce avoidable inpatient and outpatient utilization by improving member self-management skills. Through remote monitoring and self-management education provided by the AMC Health team, we expect to reduce gaps in care and help members:
- Improve medication adherence
- Improve their diet and make other lifestyle changes that may improve their conditions
- Improve communication with their physicians
Our care management team identifies members for the program, using criteria based on their diagnosis and claims. The team will refer members to the program for up to 12 months.
Patients who agree to participate in this program use simple monitoring devices to measure their vital signs (blood pressure, pulse, body weight and glucose level). Results will be compared against nationally accepted standards. Care managers follow up with patients whose results are outside the normal range.
Each participating member’s primary care physician has the ability to customize the program so that it’s consistent with their treatment plan for the patient. When members are referred to the program, AMC Health sends providers a welcome fax informing them of the patient’s participation in the program.
We’re planning to expand the program in the future for members with chronic obstructive pulmonary disorder.
Prior authorization requests for outpatient CAR-T therapy drugs for Medicare Advantage members
For dates of service on or after Jan. 1, 2021, outpatient CAR-T therapy drugs, such as Yescarta®, Kymriah® and Tecartus™, will be managed by Blue Cross Blue Shield of Michigan or Blue Care Network under the medical benefit for Medicare Plus Blue℠ and BCN Advantage℠ members. For dates of service prior to Jan. 1, 2021, CAR-T cell therapy is covered under Original Medicare.
You must submit prior authorization requests for outpatient CAR-T therapy drugs before providing services.
Submit prior authorization requests, including all relevant clinical documentation, using one of these methods:
- Enter the request in the NovoLogix® online tool. For more information about this process, see the “NovoLogix” section below.
- Fax the request to the Pharmacy Clinical Help Desk at 1-866-392-6465.
Note: Prior authorization for CAR-T drugs isn’t managed by AIM Specialty Health®.
If you have any questions, send an email to MASRX@bcbsm.com.
How to bill
For Medicare Plus Blue and BCN Advantage, we require authorization for all outpatient places of service when you bill these medications as a professional or outpatient facility service as follows:
- Electronically through an 837P transaction or on a professional CMS-1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
NovoLogix
For CAR-T therapy drugs, submit authorization requests through NovoLogix. It offers real-time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to enter authorization requests through NovoLogix.
To request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
We’ll no longer cover Cosentyx for commercial members, starting Jan. 1, 2021
Blue Cross Blue Shield of Michigan and Blue Care Network will no longer cover Cosentyx® for commercial members, starting Jan. 1, 2021, but current Cosentyx authorization for these members is effective until its expiration date or Feb. 28, 2021, whichever comes first. However, members may pay more on or after Jan. 1, 2021.
Members who fill a prescription for Cosentyx on or after March 1, 2021, will be responsible for the full cost.
Taltz® will be added as a preferred medication, effective Jan. 1, 2021. We’ll cover the following alternatives that have similar effectiveness, quality and safety:
| Common use |
Preferred alternatives |
| Ankylosing spondylitis |
Taltz, Enbrel®, Humira® |
| Psoriatic arthritis |
Taltz, Enbrel, Humira, Otezla®, Stelara® SC, Tremfya®, Xeljanz®/XR |
| Psoriasis |
Taltz, Enbrel, Humira, Otezla, Skyrizi®, Stelara SC, Tremfya |
We’ll send letters to affected members and their groups, as well as to health care providers, to let them know about this change.
Skyrizi and Tegsedi covered under pharmacy benefit for Blue Cross and BCN commercial members
We’re changing how we cover Skyrizi® and Tegsedi® for our Blue Cross Blue Shield of Michigan and Blue Care Network commercial members. As of Oct. 8, 2020, our Blue Cross and BCN commercial plans no longer cover the following medications under the medical benefit but will cover them under the pharmacy benefit:
- Skyrizi (risankizumab-rzaa), HCPCS codes C9399, J3590
- Tegsedi (inotersen), HCPCS codes C9399, J3490
Coverage for these drugs is moving to the pharmacy benefit because they can be safely and conveniently self-administered in the member’s home. We'll contact members and advise them to talk to their doctor about prescribing these medications for purchase from a pharmacy.
These drugs are available through pharmacies that dispense specialty drugs, including AllianceRx Walgreens Prime Specialty Pharmacy. Health care providers who administer these medications to their patients on or after Oct. 8, 2020, will be responsible for the cost.
There are no changes to how these therapies should be managed.
- Both Skyrizi and Tegsedi will continue to require prior authorization. (See more information on submitting prior authorization requests below.)
- For Skyrizi, quantity limits continue to apply.
- For Tegsedi, documentation requirements continue to apply.
Submitting prior authorization requests
Providers can submit prior authorization requests for these drugs as follows:
- Electronically: Through CoverMyMeds® or another free ePA tool, such as Surescripts® or ExpressPAth®. See Save time and submit your prior authorization requests electronically for pharmacy benefit drugs for more information.
- By phone: Call 1-800-437-3803.
- By fax: Call the Pharmacy Clinical Help Desk at 1-800-437-3803 to obtain the pertinent medication request form, which you can then complete and submit by fax.
- For Blue Cross commercial members: Fax the medication request form to 1-866-601-4425.
- For BCN commercial members: Fax the medication request form to 1-877-442-3778.
- By mail:
Pharmacy Services — Mail Code 512
Blue Cross Blue Shield of Michigan
600 E. Lafayette Blvd.
Detroit, MI 48226-2998
List of requirements
To view requirements for Skyrizi, Tegsedi and other drugs covered under the pharmacy benefit, see the Prior authorization and step therapy coverage criteria for Blue Cross and BCN at ereferrals.bcbsm.com.
For a list of requirements related to drugs covered under the medical benefit, see the Blue Cross and BCN utilization management medical drug list for Blue Cross PPO (commercial) and BCN HMO (commercial) members document.
We won’t be re-tiering our chiropractic commercial network again this year
To help monitor and control the cost of chiropractic care, we work with Optum®, a company that assists us and our health care providers in promoting appropriate utilization in our Blue Cross Blue Shield of Michigan commercial chiropractic network.
Due to a change in how chiropractor data between Blue Cross Blue Shield of Michigan and Optum® is exchanged, there’s been a delay in posting updated profiling data to the Optum provider website.** As a result, we won’t be re-tiering our commercial chiropractic network based on July 1, 2019, to June 30, 2020, data.
We’ll provide further communication through The Record once updated data has been posted. If you have any questions before then, contact an Optum support clinician at 952-205-3121 or a Blue Cross profiling analyst at 313-448-7371.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Update: Article on direct reimbursement to athletic trainers
In an article in the October Record, we stated that athletic trainers could receive reimbursement for physical medicine services for BCN Advantage℠ members. However, since the time of publication, the scope of this reimbursement program has changed. Athletic trainers can’t be reimbursed for physical medicine services for BCN Advantage members. Also, as a reminder, they can’t be reimbursed for services to Medicare Plus Blue℠ members. Use the following updated article as your reference on this topic.
Athletic trainers can receive direct reimbursement for physical medicine services for Blue Cross Blue Shield of Michigan’s commercial members in the Traditional and TRUST networks, as well as Blue Care Network commercial members, on or after Jan. 1, 2021. Direct reimbursement to athletic trainers for services to Medicare Plus Blue and BCN Advantage members is prohibited.
Athletic trainers can now apply to participate in Blue Cross’ commercial networks (Traditional and TRUST), as well as BCN’s commercial network. To apply, go to bcbsm.com/providers and click on Enroll to become a provider. Specific qualification requirements are identified within each agreement. All applicants to the Traditional, TRUST and BCN networks must pass a credentialing review before participation. We’ll notify applicants in writing of their approval status.
Participating athletic trainers will receive direct reimbursement for covered services within the scope of their licensure at 85% of the applicable fee schedule, minus any member deductibles and copayments. This change, effective for services provided on or after Jan. 1, affects Blue Cross and BCN plans that cover services these providers are licensed to provide. To find out if a patient has coverage, check web‑DENIS for member benefits and eligibility or call Provider Inquiry at 1‑800‑344‑8525.
Authorization requests for BCN members
For services provided to BCN commercial members, athletic trainers must submit authorization requests for physical medicine services to eviCore healthcare. There is no prior authorization required for Blue Cross commercial members.
About physical medicine benefits
Athletic trainers should tell members that the physical medicine services they provide count toward a member’s physical therapy benefits. Because physical therapy benefits are limited during a plan year, the physical medicine services provided by athletic trainers will reduce a member’s future benefits for that plan year.
Educating patients about low back pain
Evidence has shown that unnecessary or routine imaging, such as X-rays, MRIs and CT scans for low back pain, isn’t associated with improved outcomes. Low back pain usually improves within the first two weeks, according to National Committee for Quality Assurance.
Avoidable imaging can put patients at risk of unnecessary treatments, surgeries and radiation exposure. When noninvasive, conservative regimens fail and surgery or therapeutic injection appear to be the only options, imaging may be considered a necessary part of the treatment plan.
To assist providers, the Michigan Quality Improvement Consortium routinely updates guidelines for assessment, diagnosis and treatment of low back pain. To help educate patients on low back pain, unnecessary imaging and treatments, the Federal Employee Program® has created a flyer on low back pain. The American Academy of Family Physicians also provides education** for patients.
This information isn’t intended to offer professional medical advice; it’s for informational purposes only.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
FEP has resources for prenatal care and well child-visits
Prenatal care and well-child visits help your patients and their children start off on a healthy path. Pregnant women are encouraged to see their doctor regularly, especially during their first trimester, for support and information on how their bodies will change and how to manage symptoms.
Parents are encouraged to bring their children in for regular well-child visits, especially during the first 15 months of life. The American Academy of Pediatrics and Bright Futures program** recommend nine well-care visits by the time a child turns 15 months.
The Blue Cross and Blue Shield Federal Employee Program® provides benefit coverage and educational resources to support pregnant women and children. There are no out-of-pocket costs for prenatal care and well-child visits when FEP members see a Preferred provider.
FEP members can visit www.fepblue.org/maternity for helpful information on prenatal care. The Pregnancy Quick Reference Guide provides information on benefits and the importance of maternity care. And the FEP Well-Child Quick Reference Guide includes the American Academy of Pediatrics’ schedule of well-child visits to help parents stay on track.
For more information on benefits and resources, FEP members and health care providers can call Customer Service at 1-800-482-3600 or go online to www.fepblue.org.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Reminder: Inpatient admission requests submitted through e-referral may be subject to clinical review
Inpatient acute care admissions for Blue Cross Blue Shield of Michigan commercial members submitted through the e-referral system could be subject to clinical review.
This policy began on Oct. 5, 2020. Clinical review is a process that our facilities already follow for acute care admissions for our Medicare Advantage members (Medicare Plus Blue℠ and BCN Advantage℠) and Blue Care Network commercial members.
With this inpatient review program, select facilities must submit authorization requests for medical inpatient stays through the e-referral system. The stays may be reviewed and approved or pended for review by a clinician.
Behavioral health authorization requests and clinical reviews will continue according to the current process with the assigned behavioral health management company, working in conjunction with Blue Cross. However, inpatient admission authorization requests processed through New Directions will be subject to full clinical review from the first day of admission and are subject to non-approval.
We’ve let affected facilities know whether their inpatient medical and behavioral health admissions require clinical review.
Why we use clinical reviews
Clinical reviews like this have become necessary as customers look for ways to reduce the amount they spend on medical services and we try to consistently apply utilization management programs across our lines of business.
These reviews also help us ensure that the patient stays are appropriate and billed correctly, based on the patient’s diagnosis and condition.
If you’re not already familiar with the e-referral system or how the process works, refer to the Training Tools page on our ereferrals.bcbsm.com website. If you need to sign up for access to the e-referral system, refer to the Sign Up or Change a User page on the website. If you still have questions, contact your provider consultant for more information.

New clinical editing resource helps you correct billing errors
We’ve posted a new billing tips resource, titled Clinical Edits: What You Need to Do and What Documentation is Needed, in the Provider Secured Services area of bcbsm.com. It’s expected to provide a clearer understanding of the clinical edits you may encounter and how to resolve them.
To find it, follow these steps:
- After logging in to Provider Secured Services, click on BCBSM Provider Publications and Resources.
- Click on Newsletters and Resources and then on Forms.
- Under Clinical Editing Appeal Form, click on Clinical Edits: What You Need to Do and What Documentation is Needed.
Once you open the document, you can use the keyword search function (Ctrl + F) to search for keywords found on your voucher. Here’s a screenshot of a portion of the new resource:

The document provides a description of our clinical editing policies. We follow nationally recognized rules and guidelines from the Centers for Medicare & Medicaid Services, current procedural terminology codes and guidance from professional practitioner associations and societies. Note: The rules aren’t all‑encompassing and are intended to provide additional guidance and understanding of clinical edits.
The document also outlines your options for correcting billing errors. We offer advice on how to proceed to correct a claim or submit an appeal and specify the required appeal documentation.
In addition to Clinical Edits: What You Need to Do and What Documentation is Needed, you’ll find another useful document on the site: Blue Cross Commercial EX codes: Recommendations Regarding Appeal or Resubmission. It offers a description of EX codes and recommendations on whether you should appeal or resubmit.
Keep in mind that there are many online medical billing and coding resources that can help you understand correct coding guidelines. Accurate claim submission and medical record documentation are crucial to correct reimbursement.
As a reminder, clinical editing is an integral part of our payment policy and you’ll need to use the established guidelines for resolving medical and benefit policy questions.
Prior authorization requests for outpatient CAR-T therapy drugs for Medicare Advantage members
For dates of service on or after Jan. 1, 2021, outpatient CAR-T therapy drugs, such as Yescarta®, Kymriah® and Tecartus™, will be managed by Blue Cross Blue Shield of Michigan or Blue Care Network under the medical benefit for Medicare Plus Blue℠ and BCN Advantage℠ members. For dates of service prior to Jan. 1, 2021, CAR-T cell therapy is covered under Original Medicare.
You must submit prior authorization requests for outpatient CAR-T therapy drugs before providing services.
Submit prior authorization requests, including all relevant clinical documentation, using one of these methods:
- Enter the request in the NovoLogix® online tool. For more information about this process, see the “NovoLogix” section below.
- Fax the request to the Pharmacy Clinical Help Desk at 1-866-392-6465.
Note: Prior authorization for CAR-T drugs isn’t managed by AIM Specialty Health®.
If you have any questions, send an email to MASRX@bcbsm.com.
How to bill
For Medicare Plus Blue and BCN Advantage, we require authorization for all outpatient places of service when you bill these medications as a professional or outpatient facility service as follows:
- Electronically through an 837P transaction or on a professional CMS-1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
NovoLogix
For CAR-T therapy drugs, submit authorization requests through NovoLogix. It offers real-time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to enter authorization requests through NovoLogix.
To request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
Skyrizi and Tegsedi covered under pharmacy benefit for Blue Cross and BCN commercial members
We’re changing how we cover Skyrizi® and Tegsedi® for our Blue Cross Blue Shield of Michigan and Blue Care Network commercial members. As of Oct. 8, 2020, our Blue Cross and BCN commercial plans no longer cover the following medications under the medical benefit but will cover them under the pharmacy benefit:
- Skyrizi (risankizumab-rzaa), HCPCS codes C9399, J3590
- Tegsedi (inotersen), HCPCS codes C9399, J3490
Coverage for these drugs is moving to the pharmacy benefit because they can be safely and conveniently self-administered in the member’s home. We'll contact members and advise them to talk to their doctor about prescribing these medications for purchase from a pharmacy.
These drugs are available through pharmacies that dispense specialty drugs, including AllianceRx Walgreens Prime Specialty Pharmacy. Health care providers who administer these medications to their patients on or after Oct. 8, 2020, will be responsible for the cost.
There are no changes to how these therapies should be managed.
- Both Skyrizi and Tegsedi will continue to require prior authorization. (See more information on submitting prior authorization requests below.)
- For Skyrizi, quantity limits continue to apply.
- For Tegsedi, documentation requirements continue to apply.
Submitting prior authorization requests
Providers can submit prior authorization requests for these drugs as follows:
- Electronically: Through CoverMyMeds® or another free ePA tool, such as Surescripts® or ExpressPAth®. See Save time and submit your prior authorization requests electronically for pharmacy benefit drugs for more information.
- By phone: Call 1-800-437-3803.
- By fax: Call the Pharmacy Clinical Help Desk at 1-800-437-3803 to obtain the pertinent medication request form, which you can then complete and submit by fax.
- For Blue Cross commercial members: Fax the medication request form to 1-866-601-4425.
- For BCN commercial members: Fax the medication request form to 1-877-442-3778.
- By mail:
Pharmacy Services — Mail Code 512
Blue Cross Blue Shield of Michigan
600 E. Lafayette Blvd.
Detroit, MI 48226-2998
List of requirements
To view requirements for Skyrizi, Tegsedi and other drugs covered under the pharmacy benefit, see the Prior authorization and step therapy coverage criteria for Blue Cross and BCN at ereferrals.bcbsm.com.
For a list of requirements related to drugs covered under the medical benefit, see the Blue Cross and BCN utilization management medical drug list for Blue Cross PPO (commercial) and BCN HMO (commercial) members document.
Reminder: Inpatient admission requests submitted through e-referral may be subject to clinical review
Inpatient acute care admissions for Blue Cross Blue Shield of Michigan commercial members submitted through the e-referral system could be subject to clinical review.
This policy began on Oct. 5, 2020. Clinical review is a process that our facilities already follow for acute care admissions for our Medicare Advantage members (Medicare Plus Blue℠ and BCN Advantage℠) and Blue Care Network commercial members.
With this inpatient review program, select facilities must submit authorization requests for medical inpatient stays through the e-referral system. The stays may be reviewed and approved or pended for review by a clinician.
Behavioral health authorization requests and clinical reviews will continue according to the current process with the assigned behavioral health management company, working in conjunction with Blue Cross. However, inpatient admission authorization requests processed through New Directions will be subject to full clinical review from the first day of admission and are subject to non-approval.
We’ve let affected facilities know whether their inpatient medical and behavioral health admissions require clinical review.
Why we use clinical reviews
Clinical reviews like this have become necessary as customers look for ways to reduce the amount they spend on medical services and we try to consistently apply utilization management programs across our lines of business.
These reviews also help us ensure that the patient stays are appropriate and billed correctly, based on the patient’s diagnosis and condition.
If you’re not already familiar with the e-referral system or how the process works, refer to the Training Tools page on our ereferrals.bcbsm.com website. If you need to sign up for access to the e-referral system, refer to the Sign Up or Change a User page on the website. If you still have questions, contact your provider consultant for more information.
New questionnaires for Blue Cross commercial, Medicare Plus Blue plans
Starting in January 2021, Blue Cross Blue Shield of Michigan will implement new questionnaires for Blue Cross Blue Shield of Michigan commercial and Medicare Plus Blue℠ members when authorizations are entered into the e-referral system.
The questionnaires will focus on when an inpatient setting code isn’t used (e.g., *99221-*99239) for inpatient surgical procedures. Health care providers will be asked to attest that benefits and eligibility for the procedures entered have been verified. If the benefits and eligibility haven’t been verified, our utilization management team will review the authorization request and contact the facility.
These questionnaires will be similar to the questionnaires for Blue Care Network commercial and BCN Advantage℠ members.
Inpatient medical hospital peer-to-peer review request process changing for Blue Cross and BCN members
Effective Jan. 4, 2021, Blue Cross Blue Shield of Michigan will no longer accept peer-to-peer requests for Medicare Plus Blue℠ members regarding inpatient medical hospital admission denials.
Facilities are encouraged to follow the two-level provider appeal process for Medicare Plus Blue to reevaluate the denial decision on an inpatient admission request. See the “Contracted MI Provider Acute Inpatient Admission Appeals” section in the Medicare Plus Blue℠ PPO Manual.
For our Blue Care Network commercial, BCN Advantage℠ and Blue Cross commercial members, we’re still accepting peer-to-peer review requests. For these members, facilities must submit peer-to-peer review requests within seven days of the date the authorization request was denied.
We’ll update the document titled How to request a peer-to-peer review with a Blue Cross or BCN medical director to reflect the changes in the process for all lines of business. This document can be found on our ereferrals.bcbsm.com website on these webpages:
Before submitting prior authorization requests for inpatient hospital admissions, Blue Cross and BCN encourage hospitals to provide all clinical documentation needed to validate medical necessity criteria.
Here are requirements for submitting SNF and Flexible Benefit Option requests for FEP members
Before we can process Flexible Benefit Option requests and requests for skilled nursing facility admissions for Blue Cross and Blue Shield Federal Employee Program® Service Benefit Plan members, the following consents must be obtained:
Skilled Nursing Facility
Standard Option Benefit
- The member’s verbal or signed consent for the Blue Cross Blue Shield of Michigan care management program, Blue Cross® Coordinated Care, is required to process an SNF request. A signed consent must be returned by case closure.
Basic Option Benefit
- The member’s verbal or signed consent for Blue Cross® Coordinated Care is required to process a SNF request. A signed consent must be returned by case closure.
- The Flexible Benefit Option is required for SNF benefits for Basic Option members. The Flexible Benefit Option must include a signed Alternative Benefit Agreement (also known as the Member Agreement letter) before services can be approved.
- Flexible Benefit Option must include a signed Provider Agreement letter by case closure.
FEP Blue Focus benefit — 14-day maximum
- Follows the Basic Option Benefit process
Flexible Benefit Option
Used for consideration of services for approval outside the defined plan benefits, such as:
- Skilled Nursing Home benefit for Basic or FEP Blue Focus option members
- Skilled Nursing Home benefit extension for Standard Option members
- Extension of exhausted benefits such as home health care or PT/OT/ST
Before we can process Flexible Benefit Option requests, the following must be completed:
- The member’s verbal or signed care management consent for Blue Cross® Coordinated Care is required before services are provided. A signed consent must be returned by case closure.
- The Flexible Benefit Option must include a signed Alternative Benefit Agreement (also known as the Member Agreement letter) before services can be approved.
- The Flexible Benefit Option must include a signed Provider Agreement letter by case closure.
Providers can submit all required documents in one of the following ways:
- By faxing them to the Utilization Management department at 1-866-411-2573
- By attaching them to the authorization request in the e-referral system, in the Case Communication field. For instructions on how to do that, refer to the e-referral User Guide; look for “Create New (communication).”
No retrospective reviews are accepted.
New billing requirements for reduced or no-cost devices
Blue Cross Blue Shield of Michigan has adopted a new medical device billing policy to align with Centers for Medicare & Medicaid Services and industry standards.
When you bill for no-cost items due to recall, replacement or free samples, follow these guidelines:
- Charges should consistently relate to the cost of the services and uniformly apply to all patients whether inpatient or outpatient.
- Medical device hospital charges must reasonably relate to the cost of the medical device.
- When a hospital receives a replacement medical device credit, the hospital must appropriately reduce charges billed to Blue Cross.
- Bill condition codes 49, 50 or 53 to identify medical devices provided by a manufacturer for reduced or no cost.
- When billing for a device received without cost or with a credit of 50% or more toward the cost of the device, also append the “FD value” with the credited amount.
- For no-cost Items, charges billed may not exceed $1.
- For reduced items, charge should reflect any reductions as a result of the credited amount, or “FD value,” from the manufacturer.
| Coding or billing issue |
Requirement |
| What condition code do I use? |
49 — Replaced within lifespan
50 — Recalled and replaced
53 — Initially placed in a clinical trial (outpatient) |
| What value code and amount do I use? |
FD — Dollar amount of the price reduction or credit |
| How do I report a no-cost item? |
Bill charge as $0 or $1 |
You must report these condition codes on any inpatient or outpatient institutional claim that includes a no‑cost or reduced device when meeting conditions of replacement, recall or free samples. We’ll deny claim lines for “replacement, recall or free samples” when they’re not billed according to these guidelines.

We’ll add more vaccines to pharmacy benefit for commercial members
Starting Dec. 1, 2020, eligible Blue Cross Blue Shield of Michigan and Blue Care Network non-Medicare members will have coverage for additional vaccines under their pharmacy benefits plan. This allows participating pharmacies to bill through the pharmacy claims processing system.
The following hepatitis B vaccines are being added to the pharmacy benefit:
- Energix-B
- Heplisav-B
- Recombivax HB
Listed below are the vaccines (and age requirements) that will be covered under the pharmacy benefits plan:
| Vaccine |
Common name |
Age requirements |
| Influenza virus |
Flu |
None |
| Havrix® |
Hepatitis A |
None |
| Vaqta® |
Hepatitis A |
None |
| Twinrix® |
Hepatitis A and B |
None |
| Energix-B (effective Dec. 1, 2020) |
Hepatitis B |
None |
| Heplisav-B (effective Dec. 1, 2020) |
Hepatitis B |
None |
| Recombivax HB (effective Dec. 1, 2020) |
Hepatitis B |
None |
| Gardasil®9 |
HPV |
9 to 45 years old |
| M-M-R® II |
Measles, mumps, rubella |
None |
| Menveo® |
Meningitis |
None |
| Menactra® |
Meningitis |
None |
| Menomune® |
Meningitis |
None |
| Trumenba® |
Meningococcal B |
None |
| Bexsero® |
Meningococcal B |
None |
| Ipol® |
Polio |
None |
| Pneumovax 23 |
Pneumonia |
None |
Pneumococcal
(PCV7) |
Pneumonia |
None |
| Prevnar 13® |
Pneumonia |
65 and older |
| Shingrix® |
Shingles |
50 and older |
| Boostrix® |
Tetanus, diphtheria, whooping cough |
None |
| Adacel® |
Tetanus, diphtheria, whooping cough |
None |
| TDVax® |
Tetanus, diphtheria booster |
None |
| Tenivac® |
Tetanus, diphtheria booster |
None |
| Varivax® |
Varicella (chickenpox) |
None |
Vaccines for Blue Cross members can be processed under both pharmacy benefits and medical plans, but only one plan can be billed per claim. Both plans require a qualified administrator at a Blue Cross‑participating pharmacy or medical office to give the vaccine.
Qualified pharmacists giving the vaccine may bill either the member’s pharmacy benefits plan or the member’s medical plan when the pharmacy participates in the medical Vaccine Affiliation Program. Participating medical offices giving the vaccine should bill the member’s medical plan.
Most Blue Cross members with commercial prescription drug coverage are eligible for vaccines with no cost share to members if their benefits meet the coverage criteria. Grandfathered and retiree opt-out groups won’t be part of this program. These groups will maintain their current vaccine coverage under their medical benefit.
Blue Cross and BCN members can search for a participating retail pharmacy by logging in to their member account at bcbsm.com. After logging in, they would:
- Hover the mouse over My Coverage in the blue bar at the top of the page.
- Select Prescription from the drop-down menu.
Prior authorization requests for outpatient CAR-T therapy drugs for Medicare Advantage members
For dates of service on or after Jan. 1, 2021, outpatient CAR-T therapy drugs, such as Yescarta®, Kymriah® and Tecartus™, will be managed by Blue Cross Blue Shield of Michigan or Blue Care Network under the medical benefit for Medicare Plus Blue℠ and BCN Advantage℠ members. For dates of service prior to Jan. 1, 2021, CAR-T cell therapy is covered under Original Medicare.
You must submit prior authorization requests for outpatient CAR-T therapy drugs before providing services.
Submit prior authorization requests, including all relevant clinical documentation, using one of these methods:
- Enter the request in the NovoLogix® online tool. For more information about this process, see the “NovoLogix” section below.
- Fax the request to the Pharmacy Clinical Help Desk at 1-866-392-6465.
Note: Prior authorization for CAR-T drugs isn’t managed by AIM Specialty Health®.
If you have any questions, send an email to MASRX@bcbsm.com.
How to bill
For Medicare Plus Blue and BCN Advantage, we require authorization for all outpatient places of service when you bill these medications as a professional or outpatient facility service as follows:
- Electronically through an 837P transaction or on a professional CMS-1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
NovoLogix
For CAR-T therapy drugs, submit authorization requests through NovoLogix. It offers real-time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to enter authorization requests through NovoLogix.
To request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
We’ll no longer cover Cosentyx for commercial members, starting Jan. 1, 2021
Blue Cross Blue Shield of Michigan and Blue Care Network will no longer cover Cosentyx® for commercial members, starting Jan. 1, 2021, but current Cosentyx authorization for these members is effective until its expiration date or Feb. 28, 2021, whichever comes first. However, members may pay more on or after Jan. 1, 2021.
Members who fill a prescription for Cosentyx on or after March 1, 2021, will be responsible for the full cost.
Taltz® will be added as a preferred medication, effective Jan. 1, 2021. We’ll cover the following alternatives that have similar effectiveness, quality and safety:
| Common use |
Preferred alternatives |
| Ankylosing spondylitis |
Taltz, Enbrel®, Humira® |
| Psoriatic arthritis |
Taltz, Enbrel, Humira, Otezla®, Stelara® SC, Tremfya®, Xeljanz®/XR |
| Psoriasis |
Taltz, Enbrel, Humira, Otezla, Skyrizi®, Stelara SC, Tremfya |
We’ll send letters to affected members and their groups, as well as to health care providers, to let them know about this change.
Skyrizi and Tegsedi covered under pharmacy benefit for Blue Cross and BCN commercial members
We’re changing how we cover Skyrizi® and Tegsedi® for our Blue Cross Blue Shield of Michigan and Blue Care Network commercial members. As of Oct. 8, 2020, our Blue Cross and BCN commercial plans no longer cover the following medications under the medical benefit but will cover them under the pharmacy benefit:
- Skyrizi (risankizumab-rzaa), HCPCS codes C9399, J3590
- Tegsedi (inotersen), HCPCS codes C9399, J3490
Coverage for these drugs is moving to the pharmacy benefit because they can be safely and conveniently self-administered in the member’s home. We'll contact members and advise them to talk to their doctor about prescribing these medications for purchase from a pharmacy.
These drugs are available through pharmacies that dispense specialty drugs, including AllianceRx Walgreens Prime Specialty Pharmacy. Health care providers who administer these medications to their patients on or after Oct. 8, 2020, will be responsible for the cost.
There are no changes to how these therapies should be managed.
- Both Skyrizi and Tegsedi will continue to require prior authorization. (See more information on submitting prior authorization requests below.)
- For Skyrizi, quantity limits continue to apply.
- For Tegsedi, documentation requirements continue to apply.
Submitting prior authorization requests
Providers can submit prior authorization requests for these drugs as follows:
- Electronically: Through CoverMyMeds® or another free ePA tool, such as Surescripts® or ExpressPAth®. See Save time and submit your prior authorization requests electronically for pharmacy benefit drugs for more information.
- By phone: Call 1-800-437-3803.
- By fax: Call the Pharmacy Clinical Help Desk at 1-800-437-3803 to obtain the pertinent medication request form, which you can then complete and submit by fax.
- For Blue Cross commercial members: Fax the medication request form to 1-866-601-4425.
- For BCN commercial members: Fax the medication request form to 1-877-442-3778.
- By mail:
Pharmacy Services — Mail Code 512
Blue Cross Blue Shield of Michigan
600 E. Lafayette Blvd.
Detroit, MI 48226-2998
List of requirements
To view requirements for Skyrizi, Tegsedi and other drugs covered under the pharmacy benefit, see the Prior authorization and step therapy coverage criteria for Blue Cross and BCN at ereferrals.bcbsm.com.
For a list of requirements related to drugs covered under the medical benefit, see the Blue Cross and BCN utilization management medical drug list for Blue Cross PPO (commercial) and BCN HMO (commercial) members document.

New billing requirements for reduced or no-cost devices
Blue Cross Blue Shield of Michigan has adopted a new medical device billing policy to align with Centers for Medicare & Medicaid Services and industry standards.
When you bill for no-cost items due to recall, replacement or free samples, follow these guidelines:
- Charges should consistently relate to the cost of the services and uniformly apply to all patients whether inpatient or outpatient.
- Medical device hospital charges must reasonably relate to the cost of the medical device.
- When a hospital receives a replacement medical device credit, the hospital must appropriately reduce charges billed to Blue Cross.
- Bill condition codes 49, 50 or 53 to identify medical devices provided by a manufacturer for reduced or no cost.
- When billing for a device received without cost or with a credit of 50% or more toward the cost of the device, also append the “FD value” with the credited amount.
- For no-cost Items, charges billed may not exceed $1.
- For reduced items, charge should reflect any reductions as a result of the credited amount, or “FD value,” from the manufacturer.
| Coding or billing issue |
Requirement |
| What condition code do I use? |
49 — Replaced within lifespan
50 — Recalled and replaced
53 — Initially placed in a clinical trial (outpatient) |
| What value code and amount do I use? |
FD — Dollar amount of the price reduction or credit |
| How do I report a no-cost item? |
Bill charge as $0 or $1 |
You must report these condition codes on any inpatient or outpatient institutional claim that includes a no‑cost or reduced device when meeting conditions of replacement, recall or free samples. We’ll deny claim lines for “replacement, recall or free samples” when they’re not billed according to these guidelines.
|




 Blue Cross Blue Shield of Michigan and Blue Care Network will move our secure provider website to the Availity® Provider Portal in 2021. We’re going to focus on different aspects of this transition through a series of articles in this publication over the next year.
Blue Cross Blue Shield of Michigan and Blue Care Network will move our secure provider website to the Availity® Provider Portal in 2021. We’re going to focus on different aspects of this transition through a series of articles in this publication over the next year.