Subscribe | The Record Archive | Contacts | bcbsm.com
|
May 2022
Blue Cross Personal Medicine, Michigan’s first precision medicine program, introducedBlue Care Network is launching a precision medicine program, Blue Cross Personalized Medicine℠, that uses pharmacogenomics, or genetic testing, to personalize medication treatments more effectively. The idea is to give health care providers information that allows them to tailor the medication regimen of patients to their specific needs, based on review of their prescribed medications for various diagnoses. A pilot program is underway for select members through the end of this year, with a comprehensive program launch scheduled for January 2023 for eligible BCN members. It will be provided at no additional cost to members or employer group customers. “Our first priority with the Blue Cross Personalized Medicine program is to ensure that a physician is able to provide the right medication, at the right dose, as early in the process as possible,” said Dr. Scott Betzelos, vice president of HMO strategy and affordability at BCN. “This is a real opportunity to address health care on a person-by-person basis that is tailored to each member’s individual needs. Working closely with our members and their physicians, we are now able to cut out the guesswork and make informed decisions that lead to sustainable treatment options and better patient outcomes.” In addition to providing more personalized, clinically effective health care solutions, this program will also significantly decrease the risk of adverse drug reactions for patients. Adverse drug reactions are the fourth leading cause of death, are estimated to cost $136 billion annually and account for up to 7% of all hospital admissions and up to 20% of readmissions.** About pharmacogenomics Pharmacogenomics is a subgroup of precision medicine that uses an individual’s genetic makeup to guide medication treatment options, rather than using a “one-drug-fits-all” approach with therapies used to treat an entire population. BCN has contracted with OneOme, an independent precision medicine company, to facilitate the new program. OneOme will provide testing through its evidence-based RightMed® Test, which analyzes 27 genes that may affect how a patient would respond to certain medications to reduce treatment trial and error. Providers can use test results to help evaluate medications across multiple specialties, including behavioral health, oncology, pain management and cardiology, among others. Any recommendations for medication or regimen changes are optional and are to be determined and agreed upon by the pharmacist, patient and his or her prescribing physician. In a Mayo Clinic study, 90% of patients were found to have genetic variants that could affect their responsiveness to a medication. A separate Mayo Clinic study showed that pharmacogenomics helps improve a patient confidence with their drug regimens, contributing to improved medication adherence. For more information To learn more about Blue Cross Personalized Medicine, testing or pharmacogenomics, visit oneome.com/bluecarenetwork-pgx*** or call OneOme at 1-844-663-6635 (TTY: 711), Monday through Friday, 8 a.m. to 6 p.m. Eastern time. We’ll also be providing additional information about this program in the future. **Center for Education and Research on Therapeutics at Georgetown University and the Center for Drug Evaluation and Search at the Food and Drug Administration. ***Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
We’re amending all nonhospital provider agreementsTo clearly align with the disclosure requirements of the Federal Consolidated Appropriations Act, Blue Cross Blue Shield of Michigan is making changes to provider information provisions in all nonhospital provider agreements. Effective Aug. 1, 2022, these provisions will be amended as follows: Provider Information. Blue Cross Blue Shield of Michigan may disclose provider-specific information as follows: a. Pursuant to any federal, state or local statute or regulation, Blue Cross Blue Shield of Michigan and provider recognize that confidentiality does not apply in circumstances outlined in 42 USC 300gg-119, which requires that BCBSM be permitted to disclose the following information for any reason:
b. To a customer for purpose of audit and health plan administration so long as the customer agrees to restrict its use of information to these purposes and agrees not to further disclose the information. Furthermore, this section shall not be construed to restrict Blue Cross Blue Shield of Michigan from sharing all such information with its subsidiaries. c. For purposes of public reporting of benchmarks in utilization management and quality assessment initiatives, including publication in databases for use with all consumer-driven health care product, or other similar Blue Cross Blue Shield business purposes; or e. Furthermore, this section shall not be construed to restrict Blue Cross Blue Shield of Michigan from sharing all such information with any of its subsidiaries and affiliates.
We’ve amended our departicipation criteriaEffective Aug. 1, 2022, Blue Cross Blue Shield of Michigan is updating the Departicipation in the Traditional Practitioner Participation Agreement to read as follows: Departicipation Criteria Criteria under which a PRACTITIONER will be subject to departicipation include, but are not limited to, the following:
Notice: Last day for Provider Secured Services and web-DENIS is June 21
Don’t worry. Making the move to Availity Essentials is easy. Here are our recommended steps. We encourage you to take these steps now — before Provider Secured Services and web-DENIS close their doors.
Need help? Here’s where you can find it:
Availity is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Designate Availity administrator and ensure access to e-referral and Health e-Blue tools within AvailityAction item
Each organization (office, practice or facility) must have at least one Availity administrator. Administrators handle access for other Availity users; users can’t set up their own access. The provider alerts we’ve linked to below describe how to identify the Availity administrator for your organization. Be sure your Availity administrator sets up access to these tools for all users who need to obtain information for patients who have coverage through Blue Cross Blue Shield of Michigan and Blue Care Network. If your Availity administrator doesn’t take action, users of these tools won’t be able to access them through Availity and may receive error messages when they try. Setup instructions
The following videos also show the setup steps:
Additional information See the Welcome to Availity special edition newsletter to learn more about Availity. Availity is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services.
Check out our new Secure Provider Resources site
To reach the website:
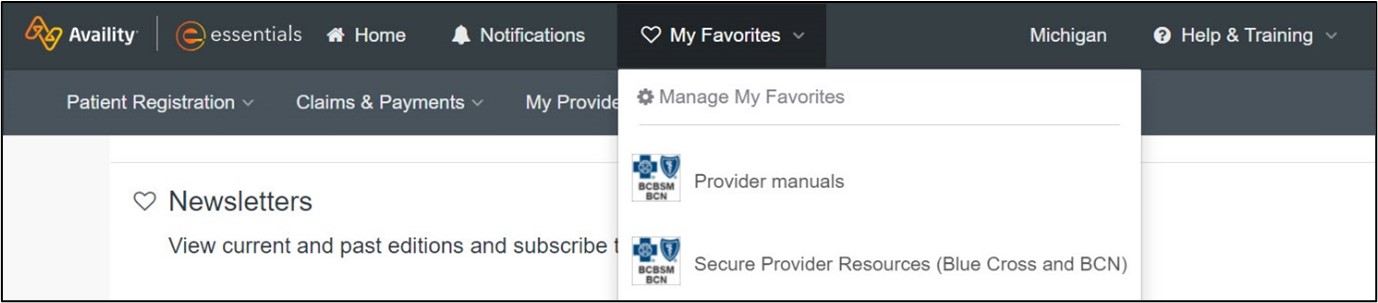
Make it a favorite You can make Secure Provider Resources a “favorite” by clicking the heart icon next to the title. When you make an item a favorite on our provider portal, you can then reach it from anywhere within the portal by clicking My Favorites at the top of the page. Any items you’ve marked as favorites throughout the portal will show up there for you to access with one click. The Secure Provider Resources site has been organized so that you can easily find the information you need. Tabs include:
Filter by plan Some pages, including Forms and Alerts, have a filter at the top to make it easier for you to find what you’re looking for. You can filter by:
Searching the site In the upper-right corner of the page, you’ll find a search box where you can search the site, including provider alerts. Enter your key words and click the magnifying glass. In the future, we plan to offer advanced search options. We encourage you to explore the new site, designed to make the information you need easy to find. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Health care providers in state’s East, Mid and Southeast regions can use new email address to reach provider consultantsBlue Cross Blue Shield of Michigan and Blue Care Network want to make it easier and simpler for you to reach a provider consultant when you need to escalate a provider inquiry. To this end, health care providers in the East, Mid and Southeast regions now have a new email address to use: petcontactus@bcbsm.com. “Pet” in the email address above stands for Provider Engagement & Transformation, or PET. It signifies that consultants want to engage with you and help ease, or transform, the way you do business with us. When you send an email to this new email address, be sure to include the following information:
Your issue will be assigned to the appropriate provider consultant, and you’ll receive status updates as your issue is resolved. The first step in resolving a question you may have continues to be contacting Provider Inquiry for any claim or benefit question. The new email address is for use when your issue isn’t resolved through Provider Inquiry. Providers in the West and Upper Peninsula regions should continue to contact their assigned consultant directly if Provider Inquiry can’t solve their claim or benefit issue. Have the Provider Inquiry reference number available when contacting a West or U.P. provider consultant as follows:
Blue Cross Commercial Provider Manual has moved to our new provider portalWhat you need to know The Blue Cross Commercial Provider Manual has moved from Benefit Explainer to our new provider portal, Availity℠. Provider manual chapters are now offered as individual PDF documents, and have been redesigned and reorganized for a more intuitive and user-friendly experience. How to access the manual If you have access to our new provider portal:
You can also access the provider manual from our Secure Provider Resources page:
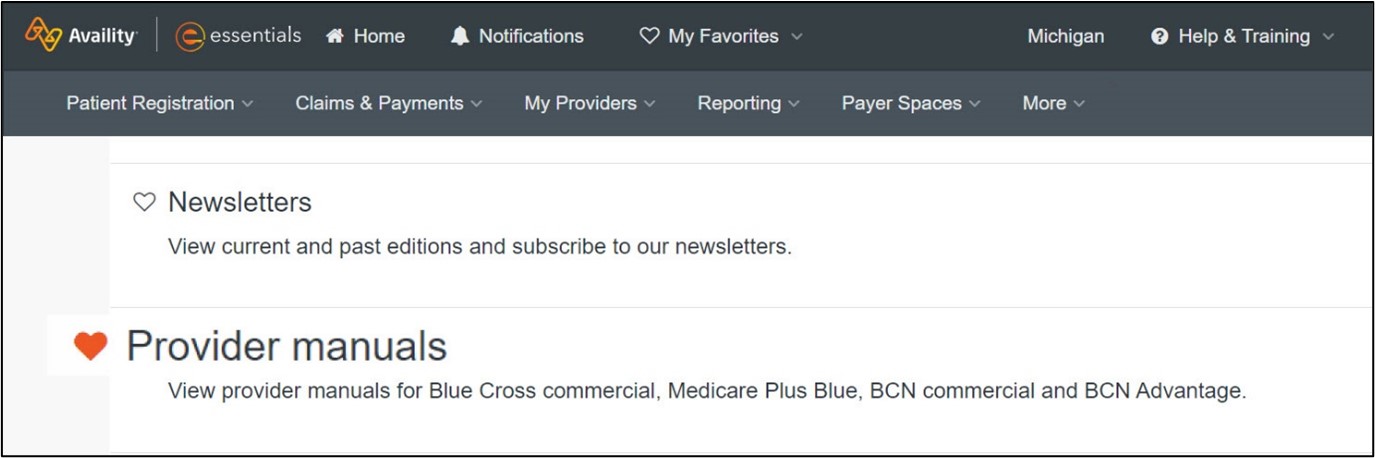
Adding resources to My Favorites Click on the heart next to Provider manuals on the Resources tab to create a shortcut in the My Favorites drop-down list on the Availity menu bar. You can also do this to add the Secure Provider Resources page to your My Favorites list. See the screenshots below for details.
If you don’t yet have access to our new provider portal, follow these steps:
Important: Access to Provider Secured Services will be ending June 21, 2022. We strongly advise registering for Availity Essentials** immediately to ensure continued access to provider materials. How to use the new provider manual Provider manual chapters have been redesigned for a more appealing, user-friendly experience, but the content within each chapter remains the same. The biggest change you’ll notice is how the chapters are presented — they’ve been reorganized to make it faster and more intuitive to find the information you need. On the Blue Cross Commercial Provider Manual webpage, you’ll see a table with topics listed on the left, and a description of the information contained within each topic on the right. To view chapters listed under a topic, simply click on a chapter name. Additional features At the top of the Blue Cross Commercial Provider Manual webpage are three bullets with links to help you use the manual. How to use the provider manual
Finding something in the manual
What's changed in the manual
Need help? For information on getting started with Availity, finding resources and accessing training, refer to the Welcome to Availity newsletter or the Welcome to Availity webpage.**
Questions or suggestions about the provider manual can be sent to ProviderManuals@bcbsm.com. Availity is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Laboratory claims editing coming to Blue Cross commercial and Medicare Plus BlueAs we wrote in a March 2022 Record article, starting in June 2022, Blue Cross Blue Shield of Michigan will be implementing a laboratory benefits management program, supported by Avalon Healthcare Solutions, for Blue Cross commercial and Medicare Plus Blue℠ claims. Avalon is an independent company that contracts with Blue Cross Blue Shield of Michigan to provide laboratory benefits management. New and revised laboratory medical policies and guidelines will take effect, which will affect certain laboratory, services, tests and procedures. To review medical policies, follow these steps:
Trial Claim Advice Tool
This tool will review claims with laboratory services for adherence and consistency with our laboratory policies, such as:
Keep in mind that this is a simulation tool and doesn’t guarantee approval or reimbursement of a claim. It’s expected that health care providers who order lab tests are doing so appropriately, according to medical necessity and relevant guidelines. Educational webinars Live educational webinars will be available to provide important information on the laboratory benefits management program, including lab policy administration, routine testing management, how to locate policy information and the trial claim advice tool. The webinar will also be recorded and made available on our provider training website. Register for one of the sessions below: May 17 – 8 to 9 a.m. Eastern time. To register, click here. Note: The appeal process won’t change. Continue to submit appeals on the Clinical Editing Appeal Form with the necessary supporting documentation. Fax one appeal at a time to avoid processing delays. **Blue Cross Blue Shield of Michigan doesn't own or control this website.
May is Mental Health Awareness MonthMay is Mental Health Awareness Month,** a good time to remind your patients of the important role that good mental health plays in overall wellness. Blue Cross Blue Shield of Michigan has developed a wide range of materials and resources focused on behavioral health — including mental health and substance use disorder — and many are suitable for sharing with patients. We encourage you to check out the following:
In addition, members can follow Blue Cross on Facebook and Twitter for regularly updated mental health information. **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Reminder: Submitting claims for behavioral health services delivered through EAPsMany companies offer their employees access to behavioral health care through employee assistance programs, or EAPs. Blue Cross Blue Shield of Michigan wants to remind our health care providers that if they provide behavioral health care services as part of one of these programs, there are different claim submission requirements to make sure that the claims are paid as part of the program. How it works
If you’re not part of the New Directions EAP network and would like to participate, you can go to ndbh.com** for more information. New Directions is an independent company that manages behavioral health services for Blue Cross Blue Shield of Michigan. **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Members can get Nonopioid Directive form on our websiteBlue Cross Blue Shield of Michigan members can obtain the Nonopioid Directive form at bcbsm.com. We’ve posted the form on our website in response to a Michigan law** that allows patients to refuse opioid medications by signing a form that their health care provider can then place in their medical records. There are four ways members will be able to access the form:
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
1st-quarter 2022 CPT code updatePathology and Laboratory/Proprietary Laboratory Analysis Codes
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
HCPCS 1st-quarter update: New and updated codesThe Centers for Medicare & Medicaid Services has added several new codes as part of its quarterly Health Care Procedure Coding System updates. The codes, effective dates and Blue Cross Blue Shield of Michigan’s coverage decisions are below. Skin substitutes
Outpatient Prospective Payment System/Surgery
Injections
Outpatient Prospective Payment System/Injections
Prosthetics and Orthotics/Medical Care
Behavioral Health
Professional /Data Gathering Codes Physician Quality Reporting Initiative
DME/POS
Behavioral Health
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
HCPCS replacement codes, effective April 1, 2022, establishedJ0219 replaces C9085, C9399, J3490, J3590 and J9999 when billing for Nexviazyme (avalglucosidase alfa-ngpt) The Centers for Medicare and Medicaid Services has established a permanent procedure code for specialty medical drug Nexviazyme (avalglucosidase alfa-ngpt). All services through March 31, 2022, will continue to be reported with code C9085, C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2022, must be reported with J0219. Prior authorization is still required for all groups opted in to the Medical Drug Prior Authorization and Site of Care program. For groups that aren’t in the Medical Drug Prior Authorization and Site of Care program this code requires manual review. J0491 replaces C9086, C9399, J3490, J3590 and J9999 when billing for Saphnelo (anifrolumab-fnia) CMS has established a permanent procedure code for specialty medical drug Saphnelo (anifrolumab-fnia). All services through March 31, 2022, will continue to be reported with code C9086, C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2022, must be reported with J0491. For groups that aren’t in the Medical Drug Prior Authorization and Site of Care program this code is covered for the FDA-approved indications. J9359 replaces C9084, C9399, J3490, J3590 and J9999 when billing for Zynlonta (loncastuximab tesirine-lpyl) CMS has established a permanent procedure code for specialty medical drugZynlonta (loncastuximab tesirine-lpyl). All services through March 31, 2022, will continue to be reported with code C9084, C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2022, must be reported with J9359. AIM prior authorization is still required for all groups opted in to the AIM Prior Authorization Program. For groups that aren’t in the AIM Prior Authorization Program this code is covered for the FDA-approved indications. J0879 replaces C9399, J3490, J3590 and J9999 when billing for Korsuva (difelikefalin) CMS has established a permanent procedure code for Korsuva (difelikefalin). All services through March 31, 2022, will continue to be reported with code C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2022, must be reported with J0879. Korsuva is covered for the following FDA-approved indication: Treatment of moderate-to-severe pruritus associated with chronic kidney disease (CKD-aP) in adults undergoing hemodialysis. J9273 replaces C9399, J3490, J3590 and J9999 when billing for Tivdak (tisotumab vedotin-tftv) CMS has established a permanent procedure code for Tivdak (tisotumab vedotin-tftv). All services through March 31, 2022, will continue to be reported with code C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2022, must be reported with J9273. Tivdak is covered for the following FDA-approved indication: Treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy. Billing chart: Blue Cross highlights medical, benefit policy changesYou’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart. This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures. You will also see that descriptions for the codes are no longer included. This is a result of recent negotiations with the AMA on use of the codes. We will publish information about new BCBS groups or changes to group benefits under the Group Benefit Changes heading. For more detailed descriptions of the BCBSM policies for these procedures, please check under the Medical/Payment Policy tab in Explainer on web-DENIS. To access this online information:
None of the information included in this billing chart is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
Review these important Collaborative Care billing tipsWe frequently receive questions about billing for our Collaborative Care Model, also known as CoCM, and wanted to share some key tips. Keep in mind that Blue Cross Blue Shield of Michigan and Blue Care Network both use billing requirements established by the Centers for Medicare & Medicaid Services. If there’s any difference between Blue Cross and CMS guidelines, the CMS information prevails.
Some practices have said they’re receiving rejections because of frequency limitations. Refer to the chart below for information on how you can avoid such denials: CoCM billing tips: Avoiding “same date” denials
For more information, check out these resources:
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Consider discussing these behavioral health resources with your patientsAs you may have read in a web-DENIS message in March, we recently published a new document titled Behavioral health resources to discuss with your patients. The document includes information about the following behavioral health resources that are available for your patients:
It’s available on the following pages of our ereferrals.bcbsm.com website:
We’re changing how we cover some prescription drugs starting in JulyWhat you need to know Our goal at Blue Cross Blue Shield of Michigan and Blue Care Network is to provide our members with safe, high-quality prescription drug therapies. We continuously review prescription drugs to provide the best value for our members, control costs and make sure our members are using the right drug for the right situation. Starting July 1, 2022, we’ll change how we cover some medications on the drug lists associated with our prescription drug plans. We’ll send letters to affected members, their groups and health care providers. Drugs that won’t be covered We’ll no longer cover the drugs on the following list. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after July 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed below, along with suggested covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
**Drug is already not covered for Preferred Drug List Drugs that will have a higher copayment The brand-name drugs that will have a higher copayment are listed, along with suggested covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. Additional coverage requirements may apply for preferred alternatives.
Brineura won’t require prior authorization for URMBT members with Blue Cross non-Medicare plansIn December 2021, we communicated that we’d be adding a prior authorization requirement for Brineura® (cerliponase alfa), HCPCS code J0567, for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, starting March 10, 2022. However, URMBT has decided not to add Brineura as a benefit for Blue Cross Blue Shield of Michigan non-Medicare plans. As a result, we updated the NovoLogix® online tool to remove prior authorization and quantity limit requirements for Brineura for these members. For information on requirements related to drugs covered under the medical benefit, refer to the document titled Medical Drug Management with Blue Cross for UAW Retiree Medical Benefit Trust PPO non-Medicare members.
Danyelza, Margenza and Saphnelo to require prior authorization for URMBT members with Blue Cross non-Medicare plansFor dates of service on or after June 30, 2022, the drugs listed below will require prior authorization for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans. They may also have site of care requirements, quantity limit requirements or both. These requirements apply when the drugs are administered in an outpatient setting. Also, they don’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714). The listed drugs are covered under the medical benefit. See the table for more information. (When a cell is blank, the drug doesn’t have that requirement.)
Submitting prior authorization requests Here’s information on how to submit requests:
To learn how to submit requests through NovoLogix:
NovoLogix offers real-time status checks and immediate approvals for certain medications. Notes:
For more information For additional information on requirements related to drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans, see:
We’ll update the pertinent drug lists to reflect the information in this article prior to the effective date. AIM Specialty Health is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services. For more information, go to our ereferrals.bcbsm.com website. **Blue Cross Blue Shield of Michigan don’t own or control this website.
Kimmtrak, Tivdak to require prior authorization for most membersFor dates of service on or after May 23, 2022, we’re adding prior authorization requirements for the following drugs covered under the medical benefit:
Prior authorization requirements apply when these drugs are administered in outpatient settings for:
Exceptions: These requirements don’t apply to Blue Cross members who have coverage through the Blue Cross and Blue Shield Federal Employee Program®, UAW Retiree Medical Benefits Trust non-Medicare members or other Blue Cross commercial members with coverage through self-funded groups.
Submit authorization requests to AIM Specialty Health® using one of the following methods:
For information about registering for and accessing the AIM ProviderPortal, refer to the Frequently asked questions page on the AIM website.** Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit, see:
We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
We’re providing purchasing and billing information for SpravatoWe’ve developed a document with information about Spravato® that we think you’ll find useful. See the document titled Spravato: Purchasing and billing information to learn about:
The document points out the differences you should be aware of when purchasing and billing Spravato for members with Blue Cross Blue Shield of Michigan commercial, Blue Care Network commercial, Medicare Plus Blue℠ and BCN Advantage℠ plans. You can access this document on these pages on our ereferrals.bcbsm.com website:
Blue Cross, BCN covering additional vaccinesTo increase access to vaccines and decrease the risk of vaccine-preventable disease outbreaks, Blue Cross Blue Shield of Michigan and Blue Care Network will add the following vaccines to our list of vaccines covered under the pharmacy benefit:
The following lists all the vaccines that are covered under eligible members’ prescription drug plans. Most Blue Cross and BCN commercial (non-Medicare) members with prescription drug coverage are eligible. If a member meets the coverage criteria, the vaccine is covered with no out-of-pocket costs.
If a member doesn’t meet the age requirement for a vaccine, Blue Cross and BCN won’t cover the vaccine under the prescription drug plan and the claim will reject. Vaccines must be administered by certified, trained and qualified registered pharmacists.
Pharmacy Opportunities Focus updated for spring 2022Pharmacy Opportunities Focus has been updated for spring 2022. The document has drug target examples based on recent, significant increases in manufacturer drug prices. Pharmacy Opportunities Focus can serve as a quick reference for multiple scenarios, such as when you’re considering drug options at the point of care or performing an evaluation of pharmacy claims data to identify high-cost drugs and their lower-cost, preferred alternatives. Below are additional suggestions to identify potential cost-saving opportunities:
The suggestions above are general guidelines. Use clinical judgment to determine the most appropriate option for each patient’s specific circumstances. For a step-by-step guide on how to navigate Health e-Blue℠ to obtain pharmacy claims data, as well as how to generate the pharmacy profile report of a primary care provider, refer to the Pharmacy Opportunities Focus: Health e-Blue Pharmacy Guide on the Health e-Blue website. Note: The information in this article applies to all members with Blue Cross Blue Shield of Michigan and Blue Care Network commercial pharmacy plans. **The drug lists are updated monthly to reflect new drug approvals, new safety or efficacy data and clinical guideline updates. Refer to the online documents for the most up-to-date versions.
Medicare sequestration resumed April 1 with a 1% reductionBlue Cross Blue Shield of Michigan and Blue Care Network are aligned with the Centers for Medicare & Medicaid Services’ guidance regarding Medicare sequestration reductions. Medicare sequestration reductions resumed April 1, 2022, with a reduction of 1% through June 30, 2022. You may recall that the U.S. Congress and President Joe Biden’s administration suspended the mandatory Medicare 2% sequestration reduction through the end of 2021. Congress passed legislation on Dec. 9, 2021, that suspended the 2% sequestration reduction through March 31, 2022, and reduced the sequestration cuts to 1% from April through June 2022. We’ll notify you through a newsletter article or a provider alert as soon as we learn the status of sequestration reductions after June 30, 2022. Reminder: The 1% reimbursement adjustment is applied after determining any applicable member deductible, copayment or other required member out-of-pocket costs. The change won’t affect reimbursement to health care providers who haven’t been affected by sequestration previously, such as providers of durable medical equipment, lab services providers and providers treating patients with end-stage renal disease.
GB Collects now handling collections of most commercial and Medicare Plus Blue claimsEffective April 1, 2022, GB Collects, a commercial collection agency, is handling the collection efforts for unreturned overpayments of most Blue Cross Blue Shield of Michigan commercial claims, as well as Medicare Plus Blue℠ claims. Commercial collection efforts This change affects all commercial claims except those for the Blue Cross and Blue Shield Federal Employee Program®. Windham Professionals will continue to handle FEP claims. Note: Collection cases that were created on or before March 31 will be handled by Windham Professionals. Medicare Plus Blue collection efforts Currently, health care providers with outstanding repayments of overpaid Medicare Plus Blue claims receive claim adjustment letters, which are system-generated, reminding them that they are 90 or 120 days past due on the repayment. The final letter states that if the debt is not settled within the following 30 days, it may be sent to collections. After we’ve exhausted all attempts to collect the debt, we’ll refer Medicare Plus Blue claims with an outstanding account receivable beyond 180 days to GB Collects for debt collection. GB Collects will conduct provider outreach and coordinate receipt of payment for Blue Cross. Once an unreturned overpayment is referred to collections, GB Collects will call or mail identified health care providers to inform them that their debt is now in collection and will request payment. GB Collects will follow up with a second and third call or letter, if necessary, if health care providers don’t respond to the initial attempts. If health care providers have questions, want details on the claim referenced in the phone call or letter, or want to pay the debt, they can call GB Collects at 1-888-688-5700. If health care providers want to dispute the claim or amount, they can refer to the “Provider dispute resolution process” outlined in the Medicare Plus Blue℠ PPO Provider Manual. GB Collects and Windham Professionals are independent companies that contract with Blue Cross Blue Shield of Michigan to provide collection services.
New resource outlines TurningPoint coding requirements for musculoskeletal procedures and related servicesTurningPoint Healthcare Solutions LLC has developed a new document titled TurningPoint Coding Requirements. It outlines the coding requirements that each prior authorization request must meet, along with examples. TurningPoint Healthcare Solutions is an independent company that manages authorizations for musculoskeletal surgical and related procedures for Blue Cross Blue Shield of Michigan and Blue Care Network. To access this new document and other key resources, see these pages of our ereferrals.bcbsm.com website:
Register now for 2022 virtual provider symposium sessionsThis year’s virtual provider symposiums run throughout May and June. Physicians, physician assistants, nurse practitioners, nurses and coders can receive continuing education credits for attending. You’re welcome to register for any session listed below. We are Stars — HEDIS®/Star measure details and exclusions: For physicians and office staff responsible for closing gaps in care related to quality adult measures.
Patient Experience — Providing great service 2.0: For physicians and office staff responsible for creating positive patient experiences. Select a date to register:
Medical record documentation and coding update: For physicians, coders, billers and administrative staff. Select a date to register:
Questions? If you have questions about the sessions, contact Ellen Kraft at ekraft@bcbsm.com. If you have registration questions, contact Patricia Scarlett at pscarlett@bcbsm.com. HEDIS®, which stands for Healthcare Effectiveness Data and Information Set, is a registered trademark of the National Committee for Quality Assurance, or NCQA. Accreditation statement: This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education through the joint providership of the Minnesota Medical Association and Blue Cross Blue Shield of Michigan. The Minnesota Medical Association is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. CME statement: The Minnesota Medical Association designates this internet live activity for a maximum of 4.5 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Lunch and learn webinars focus on risk adjustment, codingAction item Register now for webinars that can improve your coding processes. Beginning in April 2022, physicians and coders can attend webinars that provide new information on documentation and coding of common and challenging diagnoses. These live lunchtime educational sessions will include an opportunity to ask questions. Current schedule
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding registration, email Patricia Scarlett at pscarlett@bcbsm.com.
SecureCare to manage outpatient PT, OT, ST, physical medicine and chiropractic services for Blue Cross commercial and Medicare Plus BlueBlue Cross Blue Shield of Michigan has contracted with SecureCare®, an independent network performance management company, to manage the following outpatient services for Blue Cross commercial and Medicare Plus Blue℠ members, starting July 5:
SecureCare will manage these services through a retrospective clinical review program for individual providers, outpatient clinics and hospital outpatient facilities. There’s no cost to providers for this network performance management program; however, providers must participate. Action required The following provider types need to register with SecureCare to begin the performance management process and view retrospective clinical performance reports:
In the next few weeks, providers will receive a welcome packet from SecureCare that provides information on how to register with SecureCare and next steps. The packet will also include an FAQ document and other information. Additional information You can find more information about SecureCare at securecarecorp.com.** All contracting, credentialing, eligibility, benefits, member services and claims processing will remain with Blue Cross. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
AIM authorization IDs now include alpha charactersIn mid-April 2022, AIM Specialty Health® began including randomly placed alpha characters in its authorization IDs. The authorization IDs with the alpha characters are now visible in any communication involving AIM Specialty Health, including those within the AIM ProviderPortal® and the Blue Cross Blue Shield of Michigan and Blue Care Network e-referral system. This change affects all authorizations managed by AIM Specialty Health. This includes the following services: cardiology, high-tech radiology, in-lab sleep management and radiation oncology, as well as medical oncology and supportive care drugs. More details about the change Here’s more information about this change:
What’s not affected by this change This change won’t affect how determinations are made on authorization requests that AIM manages for Blue Cross commercial, Medicare Plus Blue℠, BCN commercial and BCN Advantage℠ or the claims related to them. In addition, this change doesn’t affect authorization IDs issued before the change; those will remain the same. AIM Specialty Health is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services. For more information, go to our ereferrals.bcbsm.com website.
AIM may ask for clinical information for prior authorization requestsAs part of its quality improvement efforts, AIM Specialty Health® may ask for clinical information for prior authorization requests submitted for Blue Cross Blue Shield of Michigan commercial members, starting in the third quarter of 2022. Clinical information requested will apply to:
AIM may request the additional information as part of the prior authorization process. You’ll need to submit documentation from the member’s medical record that verifies the member’s condition. AIM will review the clinical information and use it in determining the clinical appropriateness of the request. AIM is initiating this as part of their ongoing quality improvement efforts. If the information you provide doesn’t support the medical necessity of the request, AIM may deny the request. This won‘t apply to prior authorization requests submitted for Medicare Plus Blue℠ or BCN Advantage℠ members. This information is currently being requested for BCN select commercial prior authorization requests. For more information about AIM’s requirements related to services for Blue Cross commercial members, visit our Blue Cross AIM-Managed Procedures webpage at ereferrals.bcbsm.com. AIM is an independent company that manages authorization requests for high-technology radiology and other services for many Blue Cross and BCN members.
We’ll no longer accept faxed prior authorization requests for SNF admissions starting in JuneWhat you need to know Starting June 1, 2022, we'll stop accepting faxed prior authorization requests for commercial skilled nursing facility admissions. These requests must be submitted through the e-referral system. This applies to SNF requests for initial admissions and additional days for Blue Cross Blue Shield of Michigan and Blue Care Network commercial members. We previously communicated that we’d stop accepting faxed requests on Jan. 1, 2022, but we’re allowing additional time for SNFs to sign up for access to the e‑referral system and learn how to use it. Starting June 1, faxes will only be accepted for urgent requests when the e-referral system isn’t available. In those instances, fax the form using the instructions on the document titled e-referral system planned downtimes and what to do. Earlier communications We first communicated about the requirement to use the e-referral system to submit prior authorization requests for commercial SNF admissions in September 2020. This requirement went into effect Dec. 1, 2020. Since then, we’ve communicated about it several more times through web-DENIS messages, news items on our ereferrals.bcbsm.com website and articles in The Record and BCN Provider News. Sign up now to use the e-referral system Refer to our ereferrals.bcbsm.com website:
Do’s and don’ts when submitting through the e-referral system For tips on how to make it easier to use the e-referral system when submitting commercial SNF prior authorization requests, refer to the May 2021 Record article: "Do’s and don’ts when submitting commercial SNF requests using the e-referral system." Submit Medicare Advantage requests to naviHealth naviHealth, an independent company, manages prior authorization requests for SNF admissions for our Medicare Plus Blue℠ and BCN Advantage℠ members.
We’re changing how we cover some prescription drugs starting in JulyWhat you need to know Our goal at Blue Cross Blue Shield of Michigan and Blue Care Network is to provide our members with safe, high-quality prescription drug therapies. We continuously review prescription drugs to provide the best value for our members, control costs and make sure our members are using the right drug for the right situation. Starting July 1, 2022, we’ll change how we cover some medications on the drug lists associated with our prescription drug plans. We’ll send letters to affected members, their groups and health care providers. Drugs that won’t be covered We’ll no longer cover the drugs on the following list. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after July 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed below, along with suggested covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
**Drug is already not covered for Preferred Drug List Drugs that will have a higher copayment The brand-name drugs that will have a higher copayment are listed, along with suggested covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. Additional coverage requirements may apply for preferred alternatives.
Use C9399 to bill new drugs and biologicals for first year after FDA approval for Medicare Advantage membersBe sure to use HCPCS code C9399 when billing drugs and biologicals that have been approved by the U.S. Food and Drug Administration but haven’t been assigned a specific HCPCS code. C9399 should be used for new drugs and biologicals. After the first year, the code will typically be replaced by a specific code. If no specific code has been established after the first year, you should bill with one of these codes:
These instructions are based on coding guidelines published by the Centers for Medicare & Medicaid Services. They apply to Medicare Plus Blue℠ and BCN Advantage℠ members. For additional information, refer to the document titled CMS Article A55913: Billing and Coding: Hospital Outpatient Drugs and Biologicals Under the Outpatient Prospective Payment System (OPPS).** **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Empaveli, Actemra to require prior authorization for Blue Cross URMBT non-Medicare membersFor dates of service on or after June 1, 2022, we’re adding prior authorization requirements for the following drugs covered under the medical benefit:
Prior authorization requirements apply when these drugs are administered in an outpatient setting for Blue Cross Blue Shield of Michigan UAW Retiree Medical Benefits Trust non-Medicare members. These requirements don’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714). Note: For members with claims showing use of these drugs prior to June 1, we’ll automatically authorize the drugs for dates of service through Nov. 30, 2022, to provide continuity of care. You’ll need to request authorization for dates of service on or after Dec. 1, 2022. Submit prior authorization requests using the NovoLogix® tool. It offers real-time status checks and immediate approvals for certain medications. To learn how to submit requests using the NovoLogix tool, follow these steps:
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit for Blue Cross URMBT non-Medicare members, see:
Note: Accredo manages prior authorization requests for additional medical benefit drugs. We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date.
Brineura won’t require prior authorization for URMBT members with Blue Cross non-Medicare plansIn December 2021, we communicated that we’d be adding a prior authorization requirement for Brineura® (cerliponase alfa), HCPCS code J0567, for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, starting March 10, 2022. However, URMBT has decided not to add Brineura as a benefit for Blue Cross Blue Shield of Michigan non-Medicare plans. As a result, we updated the NovoLogix® online tool to remove prior authorization and quantity limit requirements for Brineura for these members. For information on requirements related to drugs covered under the medical benefit, refer to the document titled Medical Drug Management with Blue Cross for UAW Retiree Medical Benefit Trust PPO non-Medicare members.
Danyelza, Margenza and Saphnelo to require prior authorization for URMBT members with Blue Cross non-Medicare plansFor dates of service on or after June 30, 2022, the drugs listed below will require prior authorization for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans. They may also have site of care requirements, quantity limit requirements or both. These requirements apply when the drugs are administered in an outpatient setting. Also, they don’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714). The listed drugs are covered under the medical benefit. See the table for more information. (When a cell is blank, the drug doesn’t have that requirement.)
Submitting prior authorization requests Here’s information on how to submit requests:
To learn how to submit requests through NovoLogix:
NovoLogix offers real-time status checks and immediate approvals for certain medications. Notes:
For more information For additional information on requirements related to drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans, see:
We’ll update the pertinent drug lists to reflect the information in this article prior to the effective date. AIM Specialty Health is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services. For more information, go to our ereferrals.bcbsm.com website. **Blue Cross Blue Shield of Michigan don’t own or control this website.
Carvykti requires prior authorization for Medicare Advantage membersFor dates of service on or after March 7, 2022, Carvykti™ (ciltacabtagene autoleucel), HCPCS code J9999, requires prior authorization for Medicare Plus Blue℠ and BCN Advantage℠ members. Prior authorization is required for all sites of care in which this drug is administered. Submit requests for this drug using the NovoLogix® online tool. It offers real-time status checks and immediate approvals for certain medications. If you have access to the Availity® Essentials provider portal, you already have access to NovoLogix. If you need to request access to Availity, follow the instructions on the Register for web tools webpage at bcbsm.com/providers. After you’ve logged in to Availity, click on Payer Spaces and then click on the BCBSM and BCN logo. This will take you to the Blue Cross and BCN payer space, where you’ll find links to the NovoLogix tools on the Applications tab. For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members. We’ve updated the list to reflect these changes.
Kimmtrak, Tivdak to require prior authorization for most membersFor dates of service on or after May 23, 2022, we’re adding prior authorization requirements for the following drugs covered under the medical benefit:
Prior authorization requirements apply when these drugs are administered in outpatient settings for:
Exceptions: These requirements don’t apply to Blue Cross members who have coverage through the Blue Cross and Blue Shield Federal Employee Program®, UAW Retiree Medical Benefits Trust non-Medicare members or other Blue Cross commercial members with coverage through self-funded groups.
Submit authorization requests to AIM Specialty Health® using one of the following methods:
For information about registering for and accessing the AIM ProviderPortal, refer to the Frequently asked questions page on the AIM website.** Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit, see:
We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
We’re providing purchasing and billing information for SpravatoWe’ve developed a document with information about Spravato® that we think you’ll find useful. See the document titled Spravato: Purchasing and billing information to learn about:
The document points out the differences you should be aware of when purchasing and billing Spravato for members with Blue Cross Blue Shield of Michigan commercial, Blue Care Network commercial, Medicare Plus Blue℠ and BCN Advantage℠ plans. You can access this document on these pages on our ereferrals.bcbsm.com website:
Here are details on COVID-19 Test to Treat program coverageThe federal government’s new Test to Treat program provides a one-stop location for an individual to get a COVID-19 test and, if the test is positive and he or she is eligible for treatment with an oral antiviral drug such as Paxlovid™ or Molnupiravir, the prescription can be written by a health care provider and then filled, all in the same visit. Test to Treat locations can include:
Here’s what you need to know about Blue Cross Blue Shield of Michigan and Blue Care Network coverage for Test to Treat providers:
Here are resources for learning more: **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Reminder: Starting March 1, we’ve aligned our Local Rules for acute inpatient medical admissionsFor acute inpatient medical admissions of members with certain conditions, authorization requests should be submitted only after the member has spent two days in the hospital. This update to our Local Rules went into effect for all members admitted to Michigan hospitals on or after March 1, 2022. This includes Blue Cross Blue Shield of Michigan and Blue Care Network commercial members, as well as Medicare Plus Blue℠ and BCN Advantage℠ members. Note: For non-Michigan hospitals, this update applies only to Medicare Plus Blue members. About observation orders Some hospitals have asked whether an observation order is required when billing Blue Cross or BCN for observation. Blue Cross and BCN don’t require an observation order when reimbursing an observation claim. This applies to all lines of business: Blue Cross commercial, Medicare Plus Blue, BCN commercial and BCN Advantage. Additional information
We communicated about this change in our provider newsletters previously. Refer to these articles:
New resource outlines TurningPoint coding requirements for musculoskeletal procedures and related servicesTurningPoint Healthcare Solutions LLC has developed a new document titled TurningPoint Coding Requirements. It outlines the coding requirements that each prior authorization request must meet, along with examples. TurningPoint Healthcare Solutions is an independent company that manages authorizations for musculoskeletal surgical and related procedures for Blue Cross Blue Shield of Michigan and Blue Care Network. To access this new document and other key resources, see these pages of our ereferrals.bcbsm.com website:
Lunch and learn webinars focus on risk adjustment, codingAction item Register now for webinars that can improve your coding processes. Beginning in April 2022, physicians and coders can attend webinars that provide new information on documentation and coding of common and challenging diagnoses. These live lunchtime educational sessions will include an opportunity to ask questions. Current schedule
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding registration, email Patricia Scarlett at pscarlett@bcbsm.com.
SecureCare to manage outpatient PT, OT, ST, physical medicine and chiropractic services for Blue Cross commercial and Medicare Plus BlueBlue Cross Blue Shield of Michigan has contracted with SecureCare®, an independent network performance management company, to manage the following outpatient services for Blue Cross commercial and Medicare Plus Blue℠ members, starting July 5:
SecureCare will manage these services through a retrospective clinical review program for individual providers, outpatient clinics and hospital outpatient facilities. There’s no cost to providers for this network performance management program; however, providers must participate. Action required The following provider types need to register with SecureCare to begin the performance management process and view retrospective clinical performance reports:
In the next few weeks, providers will receive a welcome packet from SecureCare that provides information on how to register with SecureCare and next steps. The packet will also include an FAQ document and other information. Additional information You can find more information about SecureCare at securecarecorp.com.** All contracting, credentialing, eligibility, benefits, member services and claims processing will remain with Blue Cross. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
AIM authorization IDs now include alpha charactersIn mid-April 2022, AIM Specialty Health® began including randomly placed alpha characters in its authorization IDs. The authorization IDs with the alpha characters are now visible in any communication involving AIM Specialty Health, including those within the AIM ProviderPortal® and the Blue Cross Blue Shield of Michigan and Blue Care Network e-referral system. This change affects all authorizations managed by AIM Specialty Health. This includes the following services: cardiology, high-tech radiology, in-lab sleep management and radiation oncology, as well as medical oncology and supportive care drugs. More details about the change Here’s more information about this change:
What’s not affected by this change This change won’t affect how determinations are made on authorization requests that AIM manages for Blue Cross commercial, Medicare Plus Blue℠, BCN commercial and BCN Advantage℠ or the claims related to them. In addition, this change doesn’t affect authorization IDs issued before the change; those will remain the same. AIM Specialty Health is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services. For more information, go to our ereferrals.bcbsm.com website.
AIM may ask for clinical information for prior authorization requestsAs part of its quality improvement efforts, AIM Specialty Health® may ask for clinical information for prior authorization requests submitted for Blue Cross Blue Shield of Michigan commercial members, starting in the third quarter of 2022. Clinical information requested will apply to:
AIM may request the additional information as part of the prior authorization process. You’ll need to submit documentation from the member’s medical record that verifies the member’s condition. AIM will review the clinical information and use it in determining the clinical appropriateness of the request. AIM is initiating this as part of their ongoing quality improvement efforts. If the information you provide doesn’t support the medical necessity of the request, AIM may deny the request. This won‘t apply to prior authorization requests submitted for Medicare Plus Blue℠ or BCN Advantage℠ members. This information is currently being requested for BCN select commercial prior authorization requests. For more information about AIM’s requirements related to services for Blue Cross commercial members, visit our Blue Cross AIM-Managed Procedures webpage at ereferrals.bcbsm.com. AIM is an independent company that manages authorization requests for high-technology radiology and other services for many Blue Cross and BCN members.
We’re changing how we cover some prescription drugs starting in JulyWhat you need to know Our goal at Blue Cross Blue Shield of Michigan and Blue Care Network is to provide our members with safe, high-quality prescription drug therapies. We continuously review prescription drugs to provide the best value for our members, control costs and make sure our members are using the right drug for the right situation. Starting July 1, 2022, we’ll change how we cover some medications on the drug lists associated with our prescription drug plans. We’ll send letters to affected members, their groups and health care providers. Drugs that won’t be covered We’ll no longer cover the drugs on the following list. Unless noted, both the brand name and available generic equivalents won’t be covered. If members fill a prescription for one of these drugs on or after July 1, 2022, they’ll be responsible for the full cost. The drugs that won’t be covered are listed below, along with suggested covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. Additional coverage requirements may apply for preferred alternatives, such as prior authorization.
**Drug is already not covered for Preferred Drug List Drugs that will have a higher copayment The brand-name drugs that will have a higher copayment are listed, along with suggested covered preferred alternatives that have similar effectiveness, quality and safety. When pharmacies fill prescriptions with preferred alternatives, the generic equivalents are dispensed, if available. Additional coverage requirements may apply for preferred alternatives.
Use C9399 to bill new drugs and biologicals for first year after FDA approval for Medicare Advantage membersBe sure to use HCPCS code C9399 when billing drugs and biologicals that have been approved by the U.S. Food and Drug Administration but haven’t been assigned a specific HCPCS code. C9399 should be used for new drugs and biologicals. After the first year, the code will typically be replaced by a specific code. If no specific code has been established after the first year, you should bill with one of these codes:
These instructions are based on coding guidelines published by the Centers for Medicare & Medicaid Services. They apply to Medicare Plus Blue℠ and BCN Advantage℠ members. For additional information, refer to the document titled CMS Article A55913: Billing and Coding: Hospital Outpatient Drugs and Biologicals Under the Outpatient Prospective Payment System (OPPS).** **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Empaveli, Actemra to require prior authorization for Blue Cross URMBT non-Medicare membersFor dates of service on or after June 1, 2022, we’re adding prior authorization requirements for the following drugs covered under the medical benefit:
Prior authorization requirements apply when these drugs are administered in an outpatient setting for Blue Cross Blue Shield of Michigan UAW Retiree Medical Benefits Trust non-Medicare members. These requirements don’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714). Note: For members with claims showing use of these drugs prior to June 1, we’ll automatically authorize the drugs for dates of service through Nov. 30, 2022, to provide continuity of care. You’ll need to request authorization for dates of service on or after Dec. 1, 2022. Submit prior authorization requests using the NovoLogix® tool. It offers real-time status checks and immediate approvals for certain medications. To learn how to submit requests using the NovoLogix tool, follow these steps:
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit for Blue Cross URMBT non-Medicare members, see:
Note: Accredo manages prior authorization requests for additional medical benefit drugs. We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date.
Brineura won’t require prior authorization for URMBT members with Blue Cross non-Medicare plansIn December 2021, we communicated that we’d be adding a prior authorization requirement for Brineura® (cerliponase alfa), HCPCS code J0567, for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, starting March 10, 2022. However, URMBT has decided not to add Brineura as a benefit for Blue Cross Blue Shield of Michigan non-Medicare plans. As a result, we updated the NovoLogix® online tool to remove prior authorization and quantity limit requirements for Brineura for these members. For information on requirements related to drugs covered under the medical benefit, refer to the document titled Medical Drug Management with Blue Cross for UAW Retiree Medical Benefit Trust PPO non-Medicare members.
Danyelza, Margenza and Saphnelo to require prior authorization for URMBT members with Blue Cross non-Medicare plansFor dates of service on or after June 30, 2022, the drugs listed below will require prior authorization for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans. They may also have site of care requirements, quantity limit requirements or both. These requirements apply when the drugs are administered in an outpatient setting. Also, they don’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714). The listed drugs are covered under the medical benefit. See the table for more information. (When a cell is blank, the drug doesn’t have that requirement.)
Submitting prior authorization requests Here’s information on how to submit requests:
To learn how to submit requests through NovoLogix:
NovoLogix offers real-time status checks and immediate approvals for certain medications. Notes:
For more information For additional information on requirements related to drugs covered under the medical benefit for URMBT members with Blue Cross non-Medicare plans, see:
We’ll update the pertinent drug lists to reflect the information in this article prior to the effective date. AIM Specialty Health is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services. For more information, go to our ereferrals.bcbsm.com website. **Blue Cross Blue Shield of Michigan don’t own or control this website.
Carvykti requires prior authorization for Medicare Advantage membersFor dates of service on or after March 7, 2022, Carvykti™ (ciltacabtagene autoleucel), HCPCS code J9999, requires prior authorization for Medicare Plus Blue℠ and BCN Advantage℠ members. Prior authorization is required for all sites of care in which this drug is administered. Submit requests for this drug using the NovoLogix® online tool. It offers real-time status checks and immediate approvals for certain medications. If you have access to the Availity® Essentials provider portal, you already have access to NovoLogix. If you need to request access to Availity, follow the instructions on the Register for web tools webpage at bcbsm.com/providers. After you’ve logged in to Availity, click on Payer Spaces and then click on the BCBSM and BCN logo. This will take you to the Blue Cross and BCN payer space, where you’ll find links to the NovoLogix tools on the Applications tab. For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members. We’ve updated the list to reflect these changes.
Kimmtrak, Tivdak to require prior authorization for most membersFor dates of service on or after May 23, 2022, we’re adding prior authorization requirements for the following drugs covered under the medical benefit:
Prior authorization requirements apply when these drugs are administered in outpatient settings for:
Exceptions: These requirements don’t apply to Blue Cross members who have coverage through the Blue Cross and Blue Shield Federal Employee Program®, UAW Retiree Medical Benefits Trust non-Medicare members or other Blue Cross commercial members with coverage through self-funded groups.
Submit authorization requests to AIM Specialty Health® using one of the following methods:
For information about registering for and accessing the AIM ProviderPortal, refer to the Frequently asked questions page on the AIM website.** Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members. For additional information on requirements related to drugs covered under the medical benefit, see:
We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
We’re providing purchasing and billing information for SpravatoWe’ve developed a document with information about Spravato® that we think you’ll find useful. See the document titled Spravato: Purchasing and billing information to learn about:
The document points out the differences you should be aware of when purchasing and billing Spravato for members with Blue Cross Blue Shield of Michigan commercial, Blue Care Network commercial, Medicare Plus Blue℠ and BCN Advantage℠ plans. You can access this document on these pages on our ereferrals.bcbsm.com website:
Blue Cross, BCN covering additional vaccinesTo increase access to vaccines and decrease the risk of vaccine-preventable disease outbreaks, Blue Cross Blue Shield of Michigan and Blue Care Network will add the following vaccines to our list of vaccines covered under the pharmacy benefit:
The following lists all the vaccines that are covered under eligible members’ prescription drug plans. Most Blue Cross and BCN commercial (non-Medicare) members with prescription drug coverage are eligible. If a member meets the coverage criteria, the vaccine is covered with no out-of-pocket costs.
If a member doesn’t meet the age requirement for a vaccine, Blue Cross and BCN won’t cover the vaccine under the prescription drug plan and the claim will reject. Vaccines must be administered by certified, trained and qualified registered pharmacists.
Pharmacy Opportunities Focus updated for spring 2022Pharmacy Opportunities Focus has been updated for spring 2022. The document has drug target examples based on recent, significant increases in manufacturer drug prices. Pharmacy Opportunities Focus can serve as a quick reference for multiple scenarios, such as when you’re considering drug options at the point of care or performing an evaluation of pharmacy claims data to identify high-cost drugs and their lower-cost, preferred alternatives. Below are additional suggestions to identify potential cost-saving opportunities:
The suggestions above are general guidelines. Use clinical judgment to determine the most appropriate option for each patient’s specific circumstances. For a step-by-step guide on how to navigate Health e-Blue℠ to obtain pharmacy claims data, as well as how to generate the pharmacy profile report of a primary care provider, refer to the Pharmacy Opportunities Focus: Health e-Blue Pharmacy Guide on the Health e-Blue website. Note: The information in this article applies to all members with Blue Cross Blue Shield of Michigan and Blue Care Network commercial pharmacy plans. **The drug lists are updated monthly to reflect new drug approvals, new safety or efficacy data and clinical guideline updates. Refer to the online documents for the most up-to-date versions.
Here are details on COVID-19 Test to Treat program coverageThe federal government’s new Test to Treat program provides a one-stop location for an individual to get a COVID-19 test and, if the test is positive and he or she is eligible for treatment with an oral antiviral drug such as Paxlovid™ or Molnupiravir, the prescription can be written by a health care provider and then filled, all in the same visit. Test to Treat locations can include:
Here’s what you need to know about Blue Cross Blue Shield of Michigan and Blue Care Network coverage for Test to Treat providers:
Here are resources for learning more: **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
No portion of this publication may be copied without the express written permission of Blue Cross Blue Shield of Michigan, except that BCBSM participating health care providers may make copies for their personal use. In no event may any portion of this publication be copied or reprinted and used for commercial purposes by any party other than BCBSM.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 It’s official. Blue Cross Blue Shield of Michigan and Blue Care Network’s Provider Secured Services and web-DENIS will have their last day of operation on June 21. Beginning June 22, these tools will be retired and no longer available.
It’s official. Blue Cross Blue Shield of Michigan and Blue Care Network’s Provider Secured Services and web-DENIS will have their last day of operation on June 21. Beginning June 22, these tools will be retired and no longer available.