|
April 2022

Availity transition dates announced
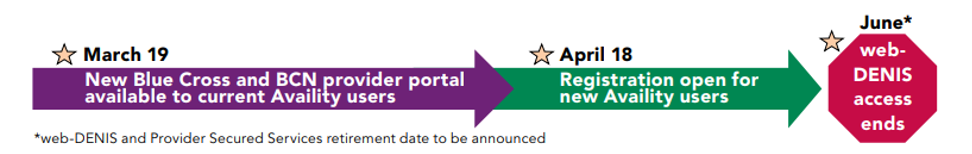
March 19, 2022 |
- If you already have access to Availity — The Blue Cross and BCN provider portal within Availity became available to you, beginning March 19, 2022. You can now use Availity for all your Blue Cross and BCN business. Check out Help & Training within Availity, and use “BCBSM” in the search tool to find Blue Cross- and BCN-specific training.
|
April 18, 2022 |
- If you don’t have access to Availity — You should register for Availity access April 18 or soon thereafter. Select an Availity administrator for your office. Have the administrator register and then add users and user roles. For details, go to Register and Get Started with Availity Essentials.** Once access is granted, begin training using the Help & Training link on the Availity homepage.
|
June 2022 |
- Access to web-DENIS ends — The last day you can use the Blue Cross and BCN Provider Secured Services and web-DENIS tools will happen in the month of June. The exact date when access will be shut down will be announced in The Record and BCN Provider News. If you don’t already receive emails for our provider newsletters, subscribe today. And make sure you’re registered for Availity, have finished training, and know how to find the tools you need within Availity before Provider Secured Services and web-DENIS are retired.
|
Welcome to Availity special edition provider newsletter
In March, you should have received a Welcome to Availity special edition provider newsletter. Please take the time to read through this issue, and share it with others in your office who need Blue Cross and BCN online information.
Questions?
If you have questions about the move to Availity Essentials, please our Frequently Asked Questions document.
Resources for the Blue Cross and BCN provider portal transition
Availity is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Blue Cross advances efforts to transform care delivery, including support for physicians
This article originally ran in the March-April issue of Hospital and Physician Update. We’re reprinting it here in case you missed it. To subscribe to the Update, a newsletter especially designed for physicians and hospital executives, click here.
For nearly 20 years, Blue Cross Blue Shield of Michigan has worked closely with health care providers to make meaningful improvements in the quality and affordability of care.
We’ve done this through such innovative initiatives as the Physician Group Incentive Program, Patient-Centered Medical Home, Collaborative Quality Initiatives and Blueprint for Affordability — all of which provide reimbursement models that reward quality and outcomes.
Despite the many successes of our joint efforts with health care partners, we recognize that we need to do even more to ensure our members get the personalized care they need — in the right setting at the right time, at the right cost. We believe value-based care models will best enable these goals and recognize we need to do more to support physicians and help them be successful in these types of arrangements.
“While the health care industry continues to experience rapid change, health plans and providers are increasingly accountable for member outcomes, cost of care and the overall experience,” said Daniel J. Loepp, Blue Cross president and CEO. “Blue Cross is addressing these expectations through stronger partnerships with our providers and working more proactively and directly with our members.”
We’re helping to meet these needs by further advancing value-based care models and transforming the way care is delivered to members. And we’re evaluating opportunities that:
- Build on our existing foundation of value-based programs.
- Offer new solutions to meet the needs of specific segments of the population.
- Partner with providers to deliver the support they need to be successful in value-based reimbursement programs.
New solutions
We began implementing several elements of this strategy last year. Here are three examples of our efforts to develop, deploy and manage new targeted solutions to meet the needs of providers and specific segments of the population:
- Helping to reduce the administrative burden of health care providers to enable success in value-based arrangements — We announced the acquisition of a management services organization in August. The MSO works with specialists to deploy clinical pathway tools and practice transformation efforts to ensure success in value-based care payment models. In addition, they offer practice management solutions, billing and payment services, and care management tools that help practices track and monitor patients’ health and coordinate their care, allowing physicians and their staffs more time to spend on patient care.
- Assisting members who need integrated, in-home care — We joined forces with Landmark Health to launch a high-intensity, in-home care program for members with multiple chronic conditions. The program offers care management, behavioral health care, medication management, 24/7 nurse triage and urgent care services to complement office-based primary care.
- Ensuring care for residents who live in underserved areas — We partnered with Dedicated Senior Medical Centers, a subsidiary of ChenMed, to establish six new primary care centers in underserved areas of Metro Detroit. The clinics will provide health care for moderate- to low-income seniors who have complex chronic conditions.
“Using deep understanding of member needs, we’ve identified areas where opportunities to offer new care models exist,” said James Grant, M.D., Blue Cross senior vice president and chief medical officer. “Now we’re developing partnerships and programs that offer defined patient populations more targeted care. This should result in better overall health for our members and reduced costs for our customers.”
He added that Blue Cross is committed to offering physicians and their staffs the tools they need to:
- Identify and close gaps in care.
- Coordinate care across the health care spectrum.
- Support practice transformation for success in value-based care models.
Market demands
All these efforts allow us to stay ahead of shifting demands in the market as we develop and offer solutions that reflect customer and member preferences, such as:
- A continued push for evolved value-based care models
- A shift in member preferences toward more convenient, cost-effective sites
“Blue Cross is well-positioned within the industry to facilitate improvements in care delivery and payment models,” said Todd Van Tol, Blue Cross executive vice president, Health Care Value. “We have comprehensive insight into members’ needs. And our strong foundation of collaboration with health care providers allows us to work together to implement new strategies and partnerships efficiently.”
Additional partnerships and programs will launch later this year, and the care delivery strategy will evolve over time in relation to the shifting needs of our members and customers.
“This is a long-term strategy,” Van Tol added. “We’ll continually work to understand member needs and monitor market conditions so we can provide care delivery models to support our population.”
You can expect to hear more about our care delivery activities in the coming months.
Newly redesigned Blue Cross Commercial Provider Manual coming later this month
The Blue Cross Commercial Provider Manual will soon complete a redesign for migration to our new provider portal. The manual will be offered as individual PDF documents for a more intuitive and user-friendly experience, aligning it with our other Blue Cross provider manuals while still being easily distinguishable.
The newly redesigned manual will be available on the Availity® provider portal in late April. You’ll be able to access the manual on Benefit Explainer until later this year. Both versions of the manual will contain identical information.
Features that are new
- Located on the Resources tab of our payer space section on our new provider portal
- Chapters available as PDF documents, providing a more intuitive and user-friendly experience
- New look and feel provides greater consistency and familiarity with other Blue Cross provider manuals
Features that have been kept or enhanced
- Chapter content and organization remain the same
- Robust tables of contents, with entries that map to each section and subsection within each chapter
- Links to other resources that provide more detail
What’s next?
Watch for more details on how to access and use the newly redesigned provider manual in the May 2022 issue of The Record.
If you have suggestions for making Blue Cross provider manuals easier to use, let us know. You can contact us at ProviderManuals@bcbsm.com.
Availity is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal services.
Update: Blue Distinction Centers for Substance Use Treatment and Recovery

As you may have read in the March-April Hospital and Physician Update, the number of designated Michigan facilities in the Blue Distinction® Centers for Substance Use Treatment and Recovery program has grown from three (when we last wrote about it in the May 2021 Record) to 14.
This BDC program is one of several initiatives implemented by Blue Cross Blue Shield of Michigan and the Blue Cross and Blue Shield Association over the past several years to battle the national opioid crisis.
The national Blue Distinction Centers for Substance Use Treatment and Recovery program aims to improve patient outcomes and value by focusing on the treatment of substance use disorders, including opioid use disorder. Facilities with residential, inpatient, intensive outpatient or partial hospitalization services are considered for this designation.
There are currently 367 designated providers across 42 states. To receive this designation, treatment facilities must offer:
- Multidisciplinary, coordinated care
- Medication-assisted treatment and other evidence-based therapies
- Nationally accredited care that recognizes specific quality standards and value-focused care
To read more, see the article in the March-April issue of Hospital and Physician Update.
Next Drug Take Back Day scheduled for April 30

The U.S. Drug Enforcement Agency’s next National Prescription Drug Take Back Day is Saturday, April 30, from 10 a.m. to 2 p.m. These twice-yearly events provide your patients with a safe, convenient and responsible way to dispose of prescription drugs. They also help educate people about the potential for abuse of medications, including opioids.
At the most recent Drug Take Back Day in October, the DEA, along with its law enforcement partners, removed nearly 745,000 pounds of unneeded prescriptions from medicine cabinets across the country.
Blue Cross Blue Shield of Michigan supports these events in various ways as part of its ongoing efforts to combat the opioid epidemic. For example, our Opioids 101 site provides a variety of resources, including flyers and brochures, that you can use to help educate your patients about opioids.
Let your patients know they can find a drug disposal facility near them that’s participating in Drug Take Back Day by checking out the DEA’s search tool.** For more information on appropriate disposal of prescription drugs, visit the DEA Diversion Control Division website**
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
No need to submit new authorization requests for Neulasta for HCPCS code J2506
On Jan. 1, 2022, the HCPCS code for Neulasta/Neulasta® Onpro® (pegfilgrastim) changed from J2505 to J2506. Health care providers who received an authorization from AIM Specialty Health® for Neulasta under HCPCS code J2505 don’t need to submit a new authorization request due to the code change.
Instead, we’ve updated the Blue Cross Blue Shield of Michigan and Blue Care Network e-referral system so that the new HCPCS code J2506 is assigned to existing Neulasta authorizations.
Check the e-referral system to confirm that your authorizations have been updated to HCPCS code 2506. This change may not be reflected in the AIM provider portal.
In the future, submit prior authorization requests for Neulasta as follows:
- For dates of service on or after Jan. 1, 2022, use HCPCS code J2506.
- For dates of service before Jan. 1, 2022, use HCPCS code J2505.
We’ve updated the following drug lists to reflect the code change:
AIM is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to provide benefit management services.
Billing chart: Blue Cross highlights medical, benefit policy changes
You’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart.
This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures.
You will also see that descriptions for the codes are no longer included. This is a result of recent negotiations with the AMA on use of the codes.
We will publish information about new BCBS groups or changes to group benefits under the Group Benefit Changes heading.
For more detailed descriptions of the BCBSM policies for these procedures, please check under the Medical/Payment Policy tab in Explainer on web-DENIS. To access this online information:
- Log in to web-DENIS.
- Click on BCBSM Provider Publications & Resources.
- Click on Benefit Policy for a Code.
- Click on Topic.
- Under Topic Criteria, click on the drop-down arrow next to Choose Identifier Type and then click on HCPCS Code.
- Enter the procedure code.
- Click on Finish.
- Click on Search.
| Code* |
BCBSM changes to:
Basic Benefit and Medical Policy, Group
Variations Payment Policy, Guidelines
|
| NEW PAYABLE PROCEDURES |
0003U, 0007U, 0016U, 0017U, 0022U, 0023U, 0026U, 0027U, 0034U, 0039U, 0040U, 0041U, 0042U, 0043U, 0044U, 0046U, 0047U, 0049U, 0064U, 0065U, 0068U, 0077U, 0093U, 0107U, 0111U, 0155U, 0177U, 0202U, 0223U, 0224U, 0226U, 0240U, 0241U
Experimental
0001U, 0002U, 0005U, 0008U, 0009U- 0014U, 0019U, 0021U, 0024U, 0025U, 0029U- 0033U, 0035U- 0038U, 0045U, 0048U, 0050U- 0056U, 0058U- 0063U, 0066U, 0067U, 0069U, 0070U- 0080U, 0082U, 0083U, 0084U, 0086U, 0087U- 0092U, 0094U-0097U, 0101U, 0102U, 0103U, 0105U, 0106U, 0108U, 0109U, 0110U, 0112U-0123U, 0129U-0138U, 0140U-0154U, 0156U-0167U, 0169U-0176U, 0178U-0201U, 0203U-0207U, 0209U-0222U, 0225U, 0227U-0239U, 0242U-0284U |
Basic benefit and medical policy
Proprietary laboratory analyses codes
Proprietary laboratory analyses, or PLA, codes
are considered experimental until the laboratory test the code represents is formally documented as established.
Established CPT codes may be used to represent and reimburse testing for incremental codes or multi-target codes.
This policy is effective March 1, 2022. |
27899,** 29999,** L8699,** 0707T
**Used to report a not otherwise classified service |
Basic benefit and medical policy
Subchondroplasty
The Subchondroplasty®, or SCP®, procedure is considered experimental.
Evidence-based conclusions regarding safety, efficacy and the effect on net health outcomes has yet to be determined. |
K1015 |
Basic benefit and medical policy
Orthotic devices
The safety and effectiveness of orthotics that are used to protect, restore, or improve all or part of an impaired body function, (e.g, braces, collars or supports) have been established.
An adjustable corrective foam foot brace with a rigid heel cup may be used on pediatric patients, effective Nov. 1, 2021. It’s used for the treatment of metatarsus adductus.
Payment policy:
Authorization requirements may apply. Claims processing may be handled by Northwood. Check member benefits for coverage details.
A maximum of two per lifetime applies (one right, one left).
Inclusions:
Guidelines are generally based on Medicare Part B and Blue Cross Blue Shield of Michigan and Blue Care Network certificate language. Specific certificate language may vary.
The orthotic device must:
- Be prescribed by a qualified health care provider
- Meet the Medicare/CMS definition of an orthotic (essentially, that it is a rigid or semi-rigid appliance, often referred to as a brace, used for the purpose of supporting or correcting a weak or deformed body part)
Orthotic devices may include, but are not limited to:
- Splints for spine, neck and shoulders
- Ankle-foot orthoses, or AFO, and knee-ankle-foot orthoses, or KAFO, for extremities
- Shoes designed for attachment to medically appropriate leg braces**
- Substitution of a somewhat different device required by change in medical condition, fit or function
- Cervical spine orthosis
- Cervical-thoracic-lumbar-sacral orthosis
- Thoracic-lumbar-sacral orthosis, or TLSO
- Lumbar orthosis
- Lumbar-sacral orthosis, or LSO
- Sacroiliac orthosis
While traveling: Orthotic devices are covered when the individual is traveling or staying at another location for a specified time period. Check individual contract/certificate language and any specific medical policy related to the item. Repair, replacement or adjustment is also covered as defined by the contract/certificate language and the applicable medical policy.
Exclusions:
Excluded orthotic devices and related services include, but are not limited to:
- Arch supports or supportive devices for the feet
- Dental appliances and bite splints
- Investigational, experimental or research devices or appliances
- Items excluded in individual certificates or riders
- Orthopedic or corrective shoes (except when either one or both are an integral part of a leg brace)**
- Orthotic devices used for participating in strenuous physical activity beyond normal activities of daily living
- Repair and replacement made necessary because of loss or damage caused by misuse or mistreatment
- Any item that is primarily made of elastic material, including, but not limited to:
- Thoracic rib belt
- Knee or kneecap orthosis, elastic, with or without stays or condylar pads, prefabricated
- Ankle orthosis, elastic, prefabricated
- Shoulder orthosis, elastic, prefabricated
- Elbow orthosis, elastic, with or without stays, prefabricated
- Wrist, hand or finger orthosis, elastic, prefabricated
**BCN only. For Blue Cross members, see BCBSM policy Orthopedic Footwear.
Specific riders may apply and override exclusions. |
| UPDATES TO PAYABLE PROCEDURES |
0161 |
Basic benefit and medical policy
New revenue code
The National Uniform Billing Committee new revenue code 0161 will be accepted effective July 1, 2022. |
Occurrence code 82 |
Basic benefit and medical policy
New occurrence code
The National Uniform Billing Committee approved new occurrence code 82, will be accepted effective July 1, 2022. |
| POLICY CLARIFICATIONS |
1111F |
Basic benefit and medical policy
Procedure code 1111F
Procedure code 1111F is changing from payable to non-payable non-payable for Blue Cross Blue Shield of Michigan commercial claims. This change is effective July 1, 2022.
Medicare Plus Blue℠ and BCN Advantage℠ will continue to separately reimburse for 1111F.
|
20552, 20553, 20605, 20606, 21010, 21050, 21060, 21070, 21073, 21085,
21116, 21240, 21242, 21243, 21480,
21485, 21490, 29800, 29804, 70328,
70330, 70332, 70336, 70350, 70355,
70486, 70487, 70488, 97010, 97024
21089, 21299, E1399, J7321, J7323-
J7326 |
Basic benefit and medical policy
Temporomandibular joint disorders
Certain tests, non-surgical and surgical procedures are considered safe and effective for the diagnosis and treatment of temporomandibular joint, or TMJ, disorders. They may be considered useful therapeutic options when indicated. This policy is effective Jan. 1, 2022.
Basic benefit policy group variations:
Procedures described in this policy don’t change member benefits for TMJ disorder. Refer to current certificate of coverage.
Inclusions:
The following diagnostic procedures when used to diagnose temporomandibular joint dysfunction:
- Diagnostic X-ray, tomograms and arthrograms
- Medical grade CT scan or MRI (generally CT scans and MRIs are reserved for presurgical evaluations)
- Cephalograms (X-rays of jaws and skull)
- Pantograms (panoramic X-rays of maxilla and mandible)
The following non-surgical treatments for the treatment of TMJ dysfunction:
- Intraoral removable prosthetic devices/appliances (encompassing fabrication, insertion, adjustment) of any and all devices/appliances constructed (excludes dental devices – see below)
- Pharmacologic treatment (such as anti-inflammatory, muscle relaxing and analgesic medications)
- Trigger-point therapy with anesthetic or corticosteroid for the treatment of myofascial pain syndrome are limited to no more than four injections in a 12-month period when all the following are met:
- There is a regional pain complaint in the expected distribution of referral pain from a trigger point.
- There is spot tenderness in a palpable taut band in a muscle.
- There is restricted range of motion.
- Conservative therapy (e.g., physical therapy, active exercises, ultrasound, heating or cooling, massage, activity modification or pharmacotherapy) doesn’t result in adequate symptom relief within two to three weeks, or isn’t feasible.
- Trigger-point injections are provided as a component of a comprehensive therapy program
The following surgical procedures for the treatment of TMJ dysfunction:
- Arthrocentesis, with or without ultrasound guidance
- Manipulation for reduction or dislocation of the TMJ
- Arthroscopic surgery in patients who objectively demonstrate (by physical examination or imaging) internal derangements (displaced discs) or degenerative joint disease and who have failed conservative treatment
- Open surgical procedures (when TMJ dysfunction results from congenital anomalies, trauma or disease in patients who have failed conservative treatment) including, but not limited to, arthroplasties, condylectomies, condylotomies, meniscus or disc plication and disc removal
Note: Dental restorations for reconstruction of tooth form and function that are a result of TMJ dysfunction or bruxism are considered a dental service and aren’t a covered medical-surgical benefit unless otherwise specified in the individual medical certificate.
Exclusions:
The following diagnostic procedures when used to diagnose bruxism** or TMJ dysfunction:
- Electromyography, or EMG, including surface EMG
- Kinesiography
- Thermography
- Neuromuscular junction testing
- Somatosensory testing
- Transcranial or lateral skull X-rays
- Intra-oral tracing or gothic arch tracing (intended to demonstrate deviations in the positioning of the jaws that are associated with TMJ dysfunction)
- Muscle testing
- Standard dental radiographic procedures
- Range of motion measurements
- Computerized mandibular scan (this measures and records muscle activity related to movement and positioning of the mandible and is intended to detect deviations in occlusion and muscle spasms related to TMJ dysfunction)
- Ultrasound/sonogram (ultrasonic Doppler auscultation)
- Arthroscopy of the TMJ for purely diagnostic purposes
- Joint vibration analysis
- Cone beam computed tomography**
- Trigger point therapy for all other indications or any medications not listed above
- Image guidance of trigger point injections
The following non-surgical procedures for the treatment of TMJ dysfunction:
- Electrogalvanic stimulation
- Iontophoresis
- Biofeedback
- Ultrasound
- Devices promoted to maintain joint range of motion and to develop muscles involved in jaw function
- Orthodontic services/treatment (e.g., dental appliance that is intended to treat malocclusion by tooth and support structure movement)
- Dental restorations/prosthesis/treatment/appliances**
- TENS, or transcutaneous electrical nerve stimulation
- PENS, or percutaneous electrical nerve stimulation
- Acupuncture
- Platelet concentrates
**Intra-oral reversible orthotic device (also known as occlusal orthotic, occlusal guard or bite splint), including fabrication, insertion and adjustment of all devices fabricated, cone beam tomography and bruxism treatment are certificate exclusions in most cases. Refer to current certificate of coverage.
|
32701, 77300, 77520, 77522, 77523, 77525
|
Basic benefit and medical policy
Charged-particle (proton or helium ion) radiotherapy
Charged-particle irradiation with proton or helium ion beams may be considered established for specific patient populations. It’s a useful therapeutic option when indicated. Inclusionary criteria have been updated, effective March 1, 2022.
If safe and effective, charged-particle irradiation with proton or helium ion beams may be open for individual consideration in the treatment of cancer based on the analysis of dosimetric data including comparative models if necessary.
Other applications of charged-particle irradiation with proton beams are considered experimental.
Note: There is insufficient evidence to show that PBRT provides an incremental benefit in the treatment of localized prostate cancer when compared to lower cost alternative procedures.
Payment policy:
Use of proton beam therapy, or PBT, may require prior authorization to verify that Blue Cross Blue Shield of Michigan and Blue Care Network criteria are met and, where appropriate, to explore the appropriateness of using alternative therapeutic modalities, such as IMRT 3-dimensional conformal radiation therapy.
Inclusions:
Charged-particle irradiation with proton or helium ion beams is established for the following indications:
- In the treatment of intracranial arteriovenous malformation not amenable to surgical excision or other conventional forms of treatment or adjacent to critical structures, such as the optic nerve, brain stem or spinal cord
- Primary or metastatic central nervous system malignancies, such as gliomas, when adjacent to critical structures such as the optic nerve, brain stem or spinal cord and when other standard radiation techniques such as IMRT or standard stereotactic modalities would not reduce the risk of radiation damage to the critical structure
- Post-operative therapy (with or without conventional high-energy X-rays) in patients who have undergone biopsy or partial resection of chordoma or low-grade (I or II), chondrosarcoma of the basisphenoid region (skull-base chordoma or chondrosarcoma), cervical spine or sacral/lower spine. Patients eligible for this treatment have residual localized tumor without evidence of metastasis.
- Tumor that involves the base of skull and proton therapy is needed to spare the orbit, optic nerve, optic chiasm or brainstem (sinonasal cancer)
- To treat melanoma of the uveal tract (including the iris, choroid or ciliary body) with no evidence of metastasis or extrascleral extension.
- In the treatment of all pediatric tumor types (through 21 years of age).
- Repeat irradiation of previously treated fields where the dose tolerance of surrounding normal structures would be exceeded with 3-D conformal radiation or IMRT
- Hepatocellular carcinoma and intrahepatic cholangiocarcinoma to treat unresectable, non-metastatic hepatocellular cancer or intrahepatic cholangiocarcinoma with curative intent
Exclusions:
- All other applications of charged-particle irradiation including, but not limited to, clinically localized prostate cancer, non-small-cell lung cancer at any stage or for recurrence, breast cancer and pancreatic cancer are experimental
- Proton beam therapy for the treatment of macular degeneration or choroidal neovascularization and hemangiomas
|
33361, 33362, 33363, 33364, 33365, 33366, 33367, 33368, 33369
Experimental:
33999**
**Unlisted procedure |
Transcatheter aortic valve implantation
Transcatheter aortic valve replacement performed with an FDA-approved transcatheter heart valve system, when performed with an approach consistent with the device’s FDA-approved labeling, may be indicated for patients with aortic stenosis.
Inclusionary and exclusionary criteria have been updated, effective March 1, 2022.
Inclusions:
Transcatheter aortic valve replacement with a device approved by the FDA and performed via an approach consistent with the device’s FDA-approved labeling is established for patients with aortic stenosis when all the following conditions are present:
- Severe aortic stenosis with a calcified aortic annulus or failure (stenosed, insufficient, or combined) of a surgical bioprosthetic aortic valve
- New York Heart Association heart failure class II, III or IV symptoms
- Left ventricular ejection fraction greater than 20% and one of the following:
- Patient isn’t an operable candidate for open surgery, as judged by at least two cardiovascular specialists including a cardiac surgeon.
- Patient is an operable candidate but is at high risk** for open surgery.
- Patient is at intermediate or greater surgical risk for open aortic valve replacement (only when used in concordance with FDA regulations for Sapien XT transcatheter heart valve; see below).
- Patient is at low surgical risk** for open aortic valve replacement (only when used in concordance with FDA regulations for Sapien 3, Sapien 3 Ultra, CoreValve Evolut R or CoreValve Evolut PRO).
Edwards SAPIEN XT transcatheter heart valve:
- Severe aortic stenosis with a calcified aortic annulus and one or more of the following:
- An aortic valve area of ≤ 1.0 cm2 or aortic valve area index ≤ 0.6 cm2/m2
- A mean aortic valve gradient ≥ 40 mmHg
- A peak aortic-jet velocity ≥ 4.0 m/sec
- Native anatomy appropriate for the 23-, 26-, or 29-mm valve system (between 18 and 28 mm)
- New York Heart Association heart failure Class II, III or IV symptoms
- Patient isn’t a candidate for open surgery, as judged by a heart team, including a cardiac surgeon, or is at high or greater risk** for open surgical therapy.
- Patient is at intermediate surgical risk** for open aortic valve replacement.
Edwards SAPIEN and Edwards SAPIEN 3 Ultra
Patient with severe aortic valve stenosis who is at low risk** for death or major complications associated with open-heart surgery
Medtronic CoreValve™ (Evolut) system
- Severe aortic stenosis with a calcified aortic annulus and one or more of the following:
- An aortic valve area of ≤ 1.0 cm2 OR aortic valve area index ≤ 0.6 cm2/m2
- A mean aortic valve gradient ≥ 40 mmHg
- A peak aortic-jet velocity ≥ 4.0 m/sec
- Native aortic annulus diameters between 23 and 31 mm
- New York Heart Association heart failure Class II, III or IV symptoms
- Patient with severe aortic valve stenosis who is at low risk or higher** for death or major complications associated with open-heart surgery
Portico™ Transcatheter Aortic Valve Implantation System
- Aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are judged by a heart team, including a cardiac surgeon, to be at high** or greater risk for open surgical therapy (e.g., predicted risk of surgical mortality ≥ 8% at 30 days, based on the Society of Thoracic Surgeons risk score and other clinical comorbidities unmeasured by the STS risk calculator).
Exclusions:
Transcatheter aortic valve replacement is considered experimental for all other indications including, but not limited to:
- The individual is appropriate candidate for the standard, open surgical approach but has refused
- Hypersensitivity or contraindication to an anticoagulation/antiplatelet regimen
- Presence of active bacterial endocarditis or other active infections
- Presence of unicuspid or bicuspid aortic valve
- Non-FDA-approved systems or approaches including:
- JenaValve systems
- Transcaval approach
|
54500, 54800, 55300, 58100, 58340,
58345, 58350, 58555, 58558, 58559,
58561, 58660, 58661, 58662, 58740,
58900, 74740, 74742, 80414, 80415,
81224, 82670, 82681, 83001, 83002, 83498, 83727, 84144, 84146, 84402,
84403, 84410, 88230, 88261, 88262,
89300, 89310, 89320, 89321, 89325,
89329, 89330, 89331, G0027, J0725 |
Basic benefit and medical policy
Infertility diagnosis
The safety and effectiveness of diagnostic testing for the evaluation of infertility have been established. These services may be considered useful in the diagnosis of a medical condition that may affect fertility. Procedure codes *88230, *88261 and *88262 were added to the policy as tests that may utilized to determine male factor infertility, effective March 1, 2022. |
76999,** 0398T, C9734
Experimental:
0071T, 0072T
**Unlisted procedure code |
Basic benefit and medical policy
MRgFUS
The safety and effectiveness of magnetic resonance-guided high-intensity ultrasound ablation have been established. It may be a considered a useful therapeutic option in specified situations.
Inclusionary criteria have been updated, effective March 1, 2022.
Inclusions:
Magnetic resonance-guided high-intensity ultrasound ablation may be considered established for the following indications:
- Pain palliation in adults with bone metastases who fail or aren’t candidates for radiotherapy
- Treatment of medication-refractory essential tremors (e.g., a failure, intolerance or contraindication to at least two trials of medication therapy)
Magnetic resonance-guided high-intensity ultrasound ablation is considered experimental in all other situations including, but not limited to:
- Treatment of uterine fibroids
- Treatment of other tumors (e.g., brain cancer, breast cancer, desmoid)
- Treatment of tremor-dominant Parkinson disease
|
78608, 78609, 78811, 78812, 78813,
78814, 78815, 78816, 78999, G0235,
A9593, A9594, A9595
Not covered:
G0219, G0252 |
Basic benefit and medical policy
PET for oncologic conditions
The safety and effectiveness of PET scanning for selected oncologic applications have been established. It’s a useful diagnostic option for patients meeting patient selection criteria. Inclusionary criteria regarding prostate cancer have been updated, effective March 1, 2022.
Payment policy:
Prior authorization may be required. Please check member benefits.
Inclusions:
All inclusionary and exclusionary statements apply to both positron emission tomography, or PET, scans and PET/computed tomography, or CT, scans, i.e., PET scans with or without PET/CT fusion.
A PET or PET/CT may be appropriate for a patient with known diagnosis of a malignancy to determine the optimal anatomic site for a biopsy or other invasive diagnostic procedure if standard imaging is equivocal. It also may replace conventional imaging when conventional imaging would be inadequate for accurate staging, and when clinical management will depend upon the stage of disease. In general, for most solid tumors, a tissue diagnosis is made prior to the performance of PET scanning. PET scans following a tissue diagnosis are performed for staging, not diagnosis. If the results of the PET scan won’t influence treatment decisions, these situations would be considered not medically necessary.
PET scans may be considered appropriate for the following oncologic conditions:
Anal cancer
- Inclusions:
- For the diagnosis, staging, restaging and monitoring of anal cancer
- Exclusions:
- Conditions not listed above
Bladder cancer
- Inclusions:
- For the staging or restaging of muscle invasive bladder cancer
- Exclusions:
- Conditions not listed above
Bone cancer
- Inclusions:
- For the staging of Ewing sarcoma and osteosarcoma
- Exclusions:
- For the staging of chondrosarcoma
Brain cancer
- Inclusions:
- For diagnosis and staging, where lesions metastatic from the brain are identified but no primary is found
- For restaging, to distinguish recurrent tumor from radiation necrosis
- Exclusions:
- Conditions not listed above
Breast cancer
- Inclusions:
- Staging and restaging of breast cancer
- Detecting locoregional or distant recurrence or metastasis (except axillary lymph nodes) when suspicion of disease is high and other imaging is inconclusive
- Staging axillary lymph nodes
- Exclusions:
- For the differential diagnosis in patients with suspicious breast lesions or an indeterminate/low suspicion finding on mammography
- For predicting pathologic response to neoadjuvant therapy for locally advanced disease.
Cancer of unknown primary
- Inclusions:
- Patients with an unknown primary who meet all the following criteria:
- In patients with a single site of disease outside the cervical lymph nodes
- Patient is considering local or regional treatment for a single site of metastatic disease
- Patient has received a negative workup for an occult primary tumor
- The PET scan will be used to rule out or detect additional sites of disease that would eliminate the rationale for local or regional treatment
- Exclusions:
- For patients with an unknown primary including, but not limited to, the following:
- As part of the initial workup of an unknown primary
- As part of the workup of patients with multiple sites of disease
Cervical cancer
- Inclusions:
- For the initial staging of patients with locally advanced cervical cancer
- For the evaluation of known or suspected recurrence
- Exclusions:
- For the initial diagnosis of cervical cancer in all other situations
Colorectal cancer
- Inclusions:
- Staging and restaging (initial and subsequent treatment strategy) to detect and assess resectability of hepatic or extrahepatic metastases of colorectal cancer
- To evaluate a rising and persistently elevated carcinoembryonic antigen, or CEA, level when standard imaging, including CT scan, is negative
- Exclusions:
- When used as a technique to assess the presence of scarring versus local bowel recurrence in patients with previously resected colorectal cancer
- When used as a technique contributing to radiotherapy treatment planning
Endometrial cancer
- Inclusions (must include both):
- Detection of lymph node metastases
- Assessment of endometrial cancer recurrence
- Exclusions:
- Conditions not listed above
Esophageal cancer
- Inclusions:
- Staging and restaging of esophageal cancer
- Determining response to preoperative induction therapy
- Exclusions:
- Detection of primary esophageal cancer
Gastric (stomach) cancer
- Inclusions:
- Diagnosis, staging and restaging of gastric carcinoma if other imaging is inconclusive
- Determining response to preoperative induction therapy
- Exclusions:
- Conditions not listed above
Head and neck cancer
- Inclusions:
- For the evaluation of the head and neck in the diagnosis of suspected head and neck cancer
- For the initial staging of the disease
- For restaging of residual or recurrent disease during follow up after treatment for their head and neck cancer
- Exclusions:
- Conditions not listed above
Hepatobiliary cancer
- Inclusions:
- When standard imaging studies are equivocal or nondiagnostic regarding extent of disease
- When standard imaging prior to planned curative surgery has been performed and has not demonstrated metastatic disease
- Exclusions:
- Conditions not listed above
Lung cancer
- Inclusions:
- Patients with a solitary pulmonary nodule as a single-scan technique (not dual-time) to distinguish between benign and malignant disease when prior CT scan and chest X-ray findings are inconclusive or discordant
- To determine resectability for patients with a presumed solitary metastatic lesion from lung cancer
- As a staging or restaging technique in those with known non-small-cell lung cancer
- PET scanning may be considered established in staging of small-cell lung cancer if limited stage is suspected based on standard imaging
- Exclusions:
- PET scanning in staging of small-cell lung cancer if extensive stage is established and in all other aspects of managing small-cell lung cancer
- Conditions not listed above
Lymphoma, including Hodgkin’s disease
- Inclusions:
- PET scanning as a technique for staging lymphoma either during initial staging or for restaging at follow-up
- Exclusions:
- Conditions not listed above
Melanoma
- Inclusions:
- Assessing extranodal spread of malignant melanoma at initial staging or at restaging during follow-up treatment for advanced disease.
- Exclusions:
- In managing stage 0, I or II melanoma
- When used as a technique to detect regional lymph node metastases in patients with clinically localized melanoma who are candidates to undergo sentinel node biopsy
Multiple myeloma
- Inclusions:
- For the initial and subsequent treatment strategy of multiple myeloma
Merkel cell carcinoma
Neuroendocrine tumors
- Inclusions:
- For the diagnosis, staging, restaging and monitoring of neuroendocrine tumors
- Exclusions:
- Conditions not listed above
Ovarian cancer
- Inclusions:
- Initial staging of ovarian cancer
- For the evaluation of patients with signs or symptoms of suspected ovarian cancer recurrence (restaging) when standard imaging, including CT scan, is inconclusive.
- Exclusions:
- For the initial evaluation (not staging) of known or suspected ovarian cancer in all other situations
Pancreatic cancer
- Inclusions:
- For the initial diagnosis and staging of pancreatic cancer when other imaging and biopsy are inconclusive
- Exclusions:
- Evaluating other aspects of pancreatic cancer
Penile cancer
- Inclusions:
- Staging inguinal lymph nodes in patients with squamous cell carcinoma of the penis.
Pleural, thymus, heart and mediastinum cancer
- Inclusions:
- For situations in which surgical resection is being considered and metastatic disease hasn’t been detected by CT or MRI
- For restaging after induction chemotherapy if the patient is a surgical candidate
Prostate cancer
- Inclusions:
- PET scanning with 11C-choline for evaluating response to primary treatment in prostate cancer
- PET scanning with Gallium Ga-68 prostate-specific membrane antigen (PSMA)-11 and Piflufolastat fluorine-18 for the following indications:
- As an alternative to standard imaging of bone and soft tissue for initial staging, for the detection of biochemically (elevated PSA) recurrent disease
- As workup for progression with bone scan plus CT or MRI for the evaluation of bone, pelvis and abdomen
- Exclusions:
- PET scanning for all other indications
Renal cell carcinoma
- Inclusions:
- For initial treatment strategy, subsequent treatment strategy and surveillance of biopsy proven kidney cancer
Soft tissue sarcoma
- Inclusions:
- For initial staging prior to resection of an apparently solitary metastasis
- When the grade of an unresectable tumor remains in doubt after biopsy
- Differentiation of suspected tumor from radiation or surgical fibrosis
- Determination of response to therapy, gastrointestinal stromal tumor, or GIST, for initial staging and re-staging when there is documented recurrence
- Exclusions:
- When used in evaluation of soft tissue sarcoma, including, but not limited to the following applications:
- Distinguishing between low grade and high- grade soft tissue sarcoma
- Detecting locoregional recurrence
- Detecting distant metastasis
Testicular cancer
- Inclusions:
- PET scanning in the evaluation of residual mass following chemotherapy of stage IIB and III seminomas
Thyroid cancer
- Inclusions:
- For the initial treatment strategy of thyroid cancer types known not to concentrate radioactive iodine, or RAI
- For subsequent treatment strategy for differentiated thyroid cancer of follicular cell origin which is known to concentrate radioactive iodine, or RAI, in all the following situations:
- When done following prior treatment with thyroidectomy and radioiodine ablation
- With a current serum thyroglobulin > 10 ng/ml (except in the setting of documented anti-thyroglobulin antibodies)
- With a negative whole-body RAI scan in the past
- Exclusions:
- For the evaluation of known or suspected differentiated or poorly differentiated thyroid cancer in all other situations
Vaginal/vulvar cancers
- Inclusions:
- Radiation planning
- Standard imaging studies are equivocal or nondiagnostic for recurrent or progressive disease
- Restaging of local recurrence when pelvic exenteration surgery is planned
Cancer surveillance
- Exclusions:
- When used as a surveillance tool for patients with cancer or with a history of cancer. A scan is considered surveillance if performed more than six months after completion of cancer therapy (12 months for lymphoma) in patients without objective signs or symptoms suggestive of cancer recurrence.
|
81161-81479, 88271, 88272, 88273, 88274, 88275, 88291, 89290, 89291
Experimental:
0254U |
Basic benefit and medical policy
Genetic testing: Preimplantation
Preimplantation genetic diagnosis may be considered established as an adjunct to in-vitro fertilization in individuals or couples who have the IVF benefit and who meet specific criteria (see inclusions).
Preimplantation genetic screening as an adjunct to IVF is considered experimental.
Inclusionary criteria have been updated, effective March 1, 2022.
Inclusions:
- For preimplantation genetic diagnosis in an embryo identified as at elevated risk of a significant genetic disorder, the individual or couple must have the benefit for in-vitro fertilization and meet criteria to access the benefit (i.e., have a diagnosis of infertility) and meet one of the following criteria:
- Both partners are known carriers of a single gene autosomal recessive disorder.
- One partner is a known carrier of a single gene autosomal recessive disorder and the partners have an offspring who has been diagnosed with that recessive disorder.
- One partner is a known carrier of a single gene autosomal dominant disorder.
- One partner is a known carrier of a single X-linked disorder.
- For preimplantation genetic diagnosis in an embryo identified as at elevated risk for a structural chromosomal abnormality, the individual or couple must have the benefit for in-vitro fertilization and meet criteria to access the benefit (e.g., have a diagnosis of infertility) and one partner with balanced or unbalanced chromosomal translocation.
- Individual consideration may be given to the individual or couple who have the in-vitro fertilization benefit and meets at least one criterion under 1. or 2. (above) but doesn’t have a diagnosis of infertility.
Exclusions:
All other situations than those specified above
Preimplantation genetic screening, or PGS, as an adjunct to IVF considered experimental |
81235, 81275, 81404, 81405, 81445,
81479, 81406 |
Basic benefit and medical policy
Non-small-cell lung cancer
Molecular analysis for targeted therapy or immunotherapy for NSCLC has gene criteria updates, effective March 1, 2022.
EGFR testing
- The safety and effectiveness of analysis of somatic variants in exons 18 (such as G719X), 19 (such as L858R, T790M), 20 (such as S678I) or 21 (such as L861Q) within the EGFR gene have been established to predict treatment response to an EGFR tyrosine kinase inhibitor, or TKI, therapy (e.g., erlotinib [Tarceva®], gefitinib [Iressa®], or afatinib [Gilotrif®]), or osimertinib (Tagrisso) in patients with advanced lung adenocarcinoma, large cell carcinoma, advanced squamous cell NSCLC and NSCLC, not otherwise specified
- The analysis for other EGFR mutations within exons 22-24 or other applications related to NSCLC is considered experimental. The peer-reviewed medical literature hasn’t yet demonstrated the clinical utility of this testing for this indication.
ALK testing
- The safety and effectiveness of analysis of somatic rearrangement mutations of the ALK gene in tissue have been established. It’s an effective diagnostic option for predicting treatment response to crizotinib (Xalkori®), ceritinib (Zykadia™, alectinib [Alecensa] or brigatinib [Alunbrig]) in patients with advanced lung adenocarcinoma and large cell carcinoma or for patients in whom an adenocarcinoma component can’t be excluded.
- Analysis of somatic rearrangement mutations of the ALK gene is considered experimental in all other situations.
BRAF V600E testing
- Analysis of the BRAF V600E variant is established to predict treatment response to BRAF or MEK inhibitor therapy (e.g., dabrafenib [Tafinlar] and trametinib [Mekinist®]) in patients with advanced lung adenocarcinoma or in whom an adenocarcinoma component cannot be excluded.
ROS1 testing
- Analysis of somatic rearrangement variants of the ROS1 gene is established to predict treatment response to ALK inhibitor therapy (crizotinib [Xalkori]) in patients with advanced lung adenocarcinoma or in whom an adenocarcinoma component can’t be excluded.
KRAS testing
- Analysis of somatic mutations of the KRAS gene is established as a technique to predict treatment nonresponse to anti-EGFR therapy with tyrosine kinase inhibitors and for the use of the anti-EGFR monoclonal antibody cetuximab in NSCLC. The peer-reviewed medical literature has demonstrated the clinical utility of this testing for this indication.
- Analysis of somatic variants of the KRAS gene in tissue may be considered established to predict treatment response to sotorasib (Lumakras) in patients with advanced lung adenocarcinoma or in whom an adenocarcinoma component can’t be excluded.
HER2 testing
- Analysis of genetic alterations in the HER2 gene for targeted therapy in patients with NSCLC is considered established to identify rare oncogenic driver variants for which effective therapy may be available.
NTRK gene fusion testing
- Analysis of gene fusions is established to predict treatment response to larotrectinib in patients with advanced lung adenocarcinoma or in whom an adenocarcinoma component can’t be excluded.
RET rearrangement testing
- Analysis of genetic alteration in the RET gene may be considered established to predict treatment response to pralsetinib or selpercatinib in patients with metastatic NSCLC.
MET exon 14 skipping alteration
- Analysis of genetic alteration that leads to MET exon 14 skipping may be considered established to predict treatment response to capmatinib in patients with metastatic NSCLC.
- Analysis of genetic alterations of the MET gene is considered experimental in all other situations.
PD-L1 testing
- PD-L1 testing may be considered established to predict treatment response to atezolizumab or to the combination of nivolumab plus ipilimumab in patients with metastatic NSCLC.
Tumor mutational burden testing
May be established for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden-high (TMB-H) [≥10 mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options (for example, Keytruda)
|
81420, 81507, 81599,** 81479***
Experimental:
81422, 0060U, 0168U
**If the codes above don’t apply and the test involves multianalyte assays and an algorithmic analysis
***If the codes above don’t apply and the test does not involve an algorithmic analysis |
Basic benefit and medical policy
Noninvasive prenatal screening for fetal aneuploidies
The safety and effectiveness of noninvasive prenatal screening for fetal aneuploidies using cell-free fetal DNA have been established. It may be considered a useful diagnostic option when indicated.
The peer-reviewed medical literature hasn’t demonstrated the clinical utility of noninvasive prenatal screening for microdeletions using cell-free fetal DNA. Therefore, this service is considered experimental.
Inclusionary and exclusionary criteria have been updated, effective March 1, 2022.
Inclusions:
- Nucleic acid sequencing-based testing of maternal plasma to screen for trisomy 21 in women with singleton and twin pregnancies (Karyotyping would be necessary to exclude the possibility of a false positive nucleic acid sequencing-based test.)
- Concurrent nucleic acid sequencing-based testing of maternal plasma for trisomy 13 and/or 18 in women who are eligible for and are undergoing nucleic acid sequencing-based testing of maternal plasma for trisomy 21
- Nucleic acid sequencing-based testing of maternal plasma for fetal sex or fetal sex chromosome aneuploidy only when certain fetal abnormalities are noted on ultrasound such as cases of ambiguous genitalia or cystic hygroma when the determination of fetal sex is necessary to help guide medical management
Exclusions:
- Nucleic acid sequencing-based testing of maternal plasma for trisomy 21 in women with multiple gestation pregnancies
- Nucleic acid sequencing-based testing of maternal plasma for trisomy 13 or 18, other than in the situations specified above
- Nucleic acid sequencing-based testing of maternal plasma for fetal sex determination or fetal sex chromosome aneuploidies other than the situation specified above
- Nucleic acid sequencing-based testing of maternal plasma for microdeletions
- Nucleic acid sequencing-based testing of maternal plasma for twin zygosity
- Vanadis® NIPT of maternal plasma to screen for trisomy 21, 18 and 13
For other aneuploidies or genetic disorders not specified above |
97039,** 97139,** 97799**
**Unlisted procedure code |
Basic benefit and medical policy
Vibracussor (percussion therapy)
Vibracussor (percussion therapy) has been added as an experimental service, effective March 1, 2022. |
J3490
J3590 |
Basic benefit and medical policy
Besremi (ropeginterferon alfa-2b-njft)
Effective Nov. 12, 2021, Besremi (ropeginterferon alfa-2b-njft) is covered for the following FDA-approved indications:
Besremi (ropeginterferon alfa-2b-njft) is an interferon alfa-2b, indicated for the treatment of adults with polycythemia vera.
Dosage and administration:
- Recommended starting dose: 100 mcg by subcutaneous injection every two weeks (50 mcg if receiving hydroxyurea).
- Increase the dose by 50 mcg every two weeks (up to a maximum of 500 mcg) until hematological parameters are stabilized.
- Interrupt or discontinue dosing if certain adverse reactions occur.
Dosage forms and strengths:
Injection: 500 mcg/mL solution in a single-dose prefilled syringe
Besremi (ropeginterferon alfa-2b-njft) isn’t a benefit for URMBT. |
J3490
J3590 |
Basic benefit and medical policy
Korsuva (difelikefalin)
Effective Aug. 23, 2021, Korsuva (difelikefalin) is covered for the following FDA-approved indications:
- Korsuva is a kappa opioid receptor agonist indicated for the treatment of moderate-to-severe pruritus associated with chronic kidney disease (CKD-aP) in adults undergoing hemodialysis, or HD.
Dosage and administration:
Recommended dosage is 0.5 mcg/kg.
- Administer by intravenous bolus injection into the venous line of the dialysis circuit at the end of each HD treatment.
- Don’t mix or dilute Korsuva prior to administration.
- Administer within 60 minutes of syringe preparation.
Dosage forms and strengths:
Injection: 65 mcg /1.3 mL (50 mcg/mL) of difelikefalin
This drug isn’t a benefit for URMBT. |
J7525 |
Basic benefit and medical policy
Prograf (tacrolimus)
Effective Aug. 1, 2021, Prograf (tacrolimus) is covered for the following updated FDA-approved indications:
- Prograf is a calcineurin-inhibitor immunosuppressant indicated for the prophylaxis of organ rejection in adult and pediatric patients receiving allogeneic liver, kidney, heart or lung transplants, in combination with other immunosuppressants.
Dosing information:
Adult lung transplant with azathioprine or MMF
Initial oral dosage (formulation): 0.075 mg/kg/day capsules, divided in two doses, every 12 hours
Whole blood trough concentration range: Months 1-3: 10-15 ng/mL; months 4-12: 8-12 ng/mL
Pediatric lung transplant
Initial oral dosage (formulation): 0.3 mg/kg/day2, capsules or oral suspension, divided in two doses, every 12 hours
Whole blood trough concentration range: Weeks 1-2: 10-20 ng/mL; Week 2 to Month 12: 10- 15 ng/mL
Dosage forms and strengths:
- Capsules: 0.5 mg, 1 mg and 5 mg
- Injection: 5 mg/ml
- For oral suspension: 0.2 mg, 1 mg unit-dose packets containing granules
|
J9177 |
Basic benefit and medical policy
Padcev (enfortumab vedotin-ejfv)
Padcev (enfortumab vedotin-ejfv) is payable for the
following updated FDA-approved indications:
Padcev is a Nectin-4-directed antibody and microtubule inhibitor conjugate indicated for the treatment of adult patients with locally advanced or metastatic urothelial cancer who:
- Are ineligible for cisplatin-containing chemotherapy and have previously received one or more prior lines of therapy
Dosage information:
- For intravenous infusion only. Don’t administer Padcev as an intravenous push or bolus. Don’t mix with or administer as an infusion with other medicinal products.
- The recommended dose of Padcev is 1.25 mg/kg (up to a maximum dose of 125 mg) given as an intravenous infusion over 30 minutes on days 1, 8 and 15 of a 28-day cycle until disease progression or unacceptable toxicity.
- Avoid use in patients with moderate or severe hepatic impairment.
|
| EXPERIMENTAL PROCEDURES |
53451, 53452, 53453, 53454 |
Basic benefit and medical policy
ProACT™ adjustable continence therapy device
The ProACT adjustable continence therapy device is experimental. It hasn’t been scientifically demonstrated to be as safe and effective as conventional treatment, effective March 1, 2022. |
None of the information included in this billing chart is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.

Blue Cross changing practitioner fees July 1
Blue Cross Blue Shield of Michigan will change practitioner fees for services with dates of service on or after July 1, 2022. This change applies to services provided to our Traditional, TRUST and Blue Preferred Plus℠ members, regardless of the customer group.
Blue Cross will use the 2022 Medicare resource-based relative value scale for most relative value unit‑priced procedures for dates of service on and after July 1. Most fees are currently priced using the 2021 values.
In addition, the conversion factor used to calculate approved amounts in Blue Cross’ anesthesia fee structure will increase to $68.78.
The Blue Cross overall fee increase of 2.25% includes base fee adjustments and value‑based reimbursement. Due to significant changes in relative value unit valuations, it’s best to review the fee schedule to view the effect on an individual code or group of codes.
Fee schedules effective July 1 will be available on web‑DENIS on April 1. To find fee schedule information, go to the homepage of web‑DENIS and follow these steps:
- Click on BCBSM Provider Publications and Resources.
- Click on Entire Fee Schedules and Fee Changes, which will bring up the End User Agreement.
- Click on Accept.
Only claims submitted with dates of service on or after July 1 will be reimbursed at the new rates.
Update: EMS providers can be reimbursed for administering monoclonal antibody COVID‑19 infusions in any location
Blue Cross Blue Shield of Michigan and Blue Care Network are reimbursing EMS providers for monoclonal antibody COVID-19 infusions administered in any location, retroactive to the effective date of the pertinent HCPCS code.
Previously, we reimbursed EMS providers for this service only when the infusions were administered in members’ homes, as we communicated in a July 2021 web-DENIS alert.
For additional information, including the effective dates for the pertinent codes, see this Feb. 18 Provider Alert.
For information about enrolling as a mass immunizer for Medicare Advantage members, see Enrollment for Administering COVID-19 Vaccines** on cms.gov.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Reminder: Don’t resubmit claims involved in audits
Action item
Once you receive a patient listing with an audit notice letter, don’t adjust the related claims any further. Also, let your billing and finance departments know which claims we’re auditing, and ask your finance team to hold off on billing any additional audit-related claims.
Blue Cross Blue Shield of Michigan requests that you not resubmit inpatient, outpatient facility, professional or ancillary claims that are already part of an audit. Simply stated, don’t rebill to correct a mistake in billing or other errors, including ones involving a CPT procedure code, ICD-10-CM diagnosis, quantity or date of service.
Rebilling audited claims causes unnecessary disruptions for all parties.
Once you receive a patient listing with an audit notice letter:
- Don’t adjust the related claims any further.
- Let your billing and finance departments know which claims we’re auditing.
- Ask your finance team to hold billing any more audit-related claims.
Audited claims could include cases for:
- Readmissions
- Diagnosis-related group
- Catastrophic-cost outliers
- Emergency room
- High-dollar claims
- Hospital physical therapy, occupational therapy and speech language pathology
- Ambulatory surgery facilities
- Freestanding outpatient physical therapy facilities
- Home health care
Audited claims could include the following types of providers:
- M.D.s, D.O.s and specialist physicians
- Certified registered nurse anesthetists
- Durable medical equipment, prosthetics and orthotics
- Home infusion therapy
- Independent physical therapy and occupational therapy
- Ophthalmology
- Oral surgery
- Pharmacy
- Podiatry
- Provider office infusion therapy
- Urgent care
For inpatient hospitalizations where the medical record doesn’t support the inpatient setting that was billed, hospitals can rebill Medicare Part B services from the denied hospitalization and be reimbursed. To learn more, see “We’re resuming Medicare Plus Blue PPO place-of-service audits” in the October 2019 Record
Provider audit suppliers conduct audits for readmissions to the same facility occurring up to 30 days from the date the patient was released from the hospital. Hospitals are responsible for all costs pertaining to readmissions denied under the Medicare Plus Blue℠ Readmissions Reimbursement Policy.
Hospitals can no longer rebill Medicare Part B services from the denied admission and can’t combine the admissions. To learn more, see “Blue Cross implements post-pay audits related to inpatient readmissions” in the January 2021 Record.
Overweight and obesity diagnoses now payable diagnosis codes
Obesity continues to be a nationwide epidemic, affecting all age groups, according to the Centers for Disease Control and Prevention.
To help address this problem, Blue Cross Blue Shield of Michigan is updating its policy to allow overweight and obesity diagnoses to be payable diagnosis codes. This change will allow providers to better diagnose and treat obesity in their patient population, improving patients’ health and well-being. It applies to all lines of business, including Blue Cross commercial, Blue Care Network commercial and our Medicare Advantage plans (Medicare Plus Blue℠ and BCN Advantage℠).
This change doesn’t affect existing benefit structures, national coverage determinations or local coverage determinations. Click here to see the list of affected codes.
Save time when submitting prior authorization requests for prescription drugs
For the fastest response to prior authorization requests for prescription drugs, use an electronic prior authorization, or ePA, tool such as CoverMyMeds® or SureScripts®.
Advantages of using an ePA tool include:
- Faster response times
- Approvals within minutes (although some requests require more time)
- Reduced administrative time
- Streamlined questions, asking only those needed for the authorization
- The ability to attach documentation if required
- Clinical criteria to guide you in submitting the proper information
- Secure and efficient prior authorization administration all in one place
- The capability of renewing existing authorizations up to 60 days before they expire
For information about submitting electronic prior authorization requests, see the following documents:
Do we have your medical records request address?
In a November 2021 Record article, we let you know that we will be working with Optum to implement some new edits. Optum is an independent company that contracts with Blue Cross Blue Shield of Michigan to identify professional and facility claims that require additional review.
One component of our relationship with Optum involves medical records requests.
To prevent claim processing delays, we need to keep your medical records request address on file. If you haven’t provided it yet or need to make changes, use the Enrollment and Change Self-Service Tool in the provider portal.
If we don’t have a medical record request address on file, the request will be sent to the mailing address. If there is no mailing address on file, the request will be sent to the billing address. If there is no billing address on file, the request will be sent to the address shown on the claim.
Outpatient PT, OT services no longer require prior authorization for Medicare Plus Blue members
For dates of service on or after April 1, 2022, Medicare Plus Blue℠ will no longer require prior authorization for outpatient physical and occupational therapy services. You can submit retroactive authorization requests to eviCore healthcare®** through July 29, 2022, for dates of service prior to April 1, 2022.
We’ll update the Medicare Plus Blue PPO Provider Manual and other documents to reflect this change.
These services will be managed by a network performance management company later this year. We’ll publish a provider alert and an article in a future issue of The Record with more details.
**eviCore healthcare is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services. For more information, go to our ereferrals.bcbsm.com website.
Reminder: RC Claim Assist available through Provider Secured Services
RC Claim Assist is a web‑based resource available to Blue Cross Blue Shield of Michigan and Blue Care Network participating providers who bill for drugs covered under the medical benefit.
This resource provides an overview of medical drug products and a calculation tool. The calculation tool identifies:
- The correct National Drug Code and CPT codes to bill
- The correct NDC quantity, unit of measure and HCPCS billable units, according to the package information
As a reminder, RC Claim Assist is available, free of charge, through Provider Secured Services.
To access RC Claim Assist:
- Log in to bcbsm.com as a provider.
- Click on the RC Claim Assist link on the Provider Secured Services welcome page.
- Follow the prompts.
Clarification: Billing for IOP services provided via telemedicine for some members
We originally communicated about this in a November 2021 Record article. The information in this article is intended to clarify billing instructions for intensive outpatient program, or IOP, services provided via telemedicine.
For behavioral health IOP services provided via telemedicine with dates of service on or after Oct. 1, 2021, bill revenue code 0905 or 0906, the applicable procedure code and modifier GT or 95. We use these modifiers to identify IOP services that are delivered via telemedicine.
Follow this guidance when billing for behavioral health IOP services provided to Blue Cross commercial, BCN commercial and BCN Advantage℠ members via telemedicine. For information about billing IOP services provided to Medicare Plus Blue℠ members via telemedicine, follow guidance from the Centers for Medicare & Medicaid Services.
Keep the following in mind:
- For Blue Cross commercial members, most plans don't cover IOP services for mental health disorders. IOP services for substance use disorders must be delivered by a substance abuse treatment facility. Be sure to check member eligibility and benefits before performing services.
- Facilities can provide IOP services to BCN commercial and BCN Advantage℠ members only when their contracts specifically include IOP services.
We’ve updated the following documents to reflect this change:
- Telehealth for behavioral health providers
- Billing tips for COVID-19 at a glance
You can find these documents on our public website at bcbsm.com/coronavirus or within Provider Secured Services.
Note: For Medicare Plus Blue members, see the Medicare-covered telehealth services for the COVID-19 PHE document to determine which IOP procedures codes are billable for telemedicine.
Requirements changed for some commercial medical benefit drugs
Blue Cross Blue Shield of Michigan and Blue Care Network encourage proper utilization of high-cost medications that are covered under the medical benefit. As part of this effort, we maintain a comprehensive list of requirements for Blue Cross and BCN group and individual commercial members.
In the October 2021 through March 2022 time frame, we added either prior authorization requirements or site-of-care requirements — or both — for Blue Cross commercial and BCN commercial members for the following medical benefit drugs:
HCPCS code |
Brand name |
Generic name |
J3590** |
Susvimo® |
ranibizumab injection, for ocular implant |
J3590** |
Cortrophin™ |
corticotrophin |
J3590** |
Vyvgart™ |
efgartigimod alfa-fcab |
J3590** |
Leqvio® |
inclisiran |
J3590** |
Tezspire™ |
tezepelumab-ekko |
J3590** |
Vabysmo™ |
faricimab-svoa |
**Will become a unique code
See the Blue Cross and BCN utilization management medical drug list for additional details. This list is available on the following pages of the ereferrals.bcbsm.com website:
As a reminder, an authorization approval isn’t a guarantee of payment. Health care providers need to verify eligibility and benefits for members.
Additional information
For Blue Cross commercial groups, this authorization requirement applies only to groups that currently participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. A link to this list is also available on the Blue Cross Medical Benefit Drugs page of the ereferrals.bcbsm.com website.
Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don't participate in the standard prior authorization program.
Enjaymo, Vabysmo and Byooviz to require prior authorization for Medicare Advantage members
Providers must submit prior authorization requests through the NovoLogix® online tool for the following drugs covered under the medical benefit for our Medicare Advantage members:
- For dates of service on or after March 7, 2022:
- Enjaymo™ (sutimlimab-jome), HCPCS code J3590
- Vabysmo™ (faricimab-svoa), HCPCS code J3590
- For dates of service on or after June 6, 2022:
- Byooviz® (ranibizumab-nuna), HCPCS code J3590
This requirement applies to Medicare Plus Blue℠ and BCN Advantage℠ members.
When prior authorization is required
We require prior authorization when these drugs are administered in any site of care other than inpatient hospital (place of service code 21) and are billed as follows:
- Electronically through an 837P transaction or on a professional CMS-1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
Submitting prior-authorization requests
Submit prior authorization requests for these drugs through NovoLogix. It offers real-time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to NovoLogix.
If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
We'll update the list to reflect these changes prior to the effective date.
Nyvepria, Rybrevant to require prior authorization for URMBT members with Blue Cross non-Medicare plans
For dates of service on or after May 16, 2022, we're adding prior authorization requirements for the following drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans:
- Nyvepria™ (pegfilgrastim-apgf), HCPCS code Q5122
- Rybrevant™ (amivantamab-vmjw), HCPCS code J9061
Submit prior authorization requests through AIM Specialty Health®.
Notes:
- The prior authorization requirement applies when these drugs are administered in an outpatient setting.
- This requirement doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714).
How to submit authorization requests
Submit prior authorization requests to AIM using one of the following methods:
More about the authorization requirements
Authorization isn't a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, see:
We’ll update the pertinent drug lists to reflect the information in this message prior to the effective date.
Accredo manages prior authorization requests for other medical benefit drugs.
AIM is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to provide benefit management services.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
We’ve updated payment policy for Unna boot or multilayer compression dressing
Blue Cross Blue Shield of Michigan has updated its payment policy to align with the Centers for Medicare & Medicaid Services’ National Correct Coding Initiative guidelines for the application of Unna boot or multilayer compression dressing when reported with a wound debridement procedure.
CPT codes *29580 (Unna boot) and *29581 (application of multilayer compression system) aren’t separately payable when applied at the same site or extremity where CPT codes *11042-*11047 or *97597-*97598 wound debridement is performed. This payment policy aligns with NCCI procedure-to-procedure coding edits and statements contained in the NCCI Policy Manual for Medicare Services.
TurningPoint authorizations for musculoskeletal surgical and related procedures are valid for 6 months
Prior authorization requests approved by TurningPoint Healthcare Solutions LLC on or after Jan. 1, 2022, are valid for six months from the planned date of service for all outpatient musculoskeletal procedures, including pain management procedures.
For example, if TurningPoint approves an authorization request for a service planned for June 1, the approval will be valid from June 1 through Nov. 30. If the surgery is performed during that time period, the authorization will match the claim without any changes to the authorization.
We updated the following documents to reflect this change:
Note: Prior authorization requests that were approved on or before Dec. 31, 2021, are valid for 30 days from the planned date of service.
TurningPoint Healthcare Solutions LLC is an independent company that manages authorizations for musculoskeletal surgical and related procedures for Blue Cross Blue Shield of Michigan and Blue Care Network.
Musculoskeletal procedure authorizations: Reminders for facilities
This article provides a review of how to change the place of service from outpatient to inpatient for musculoskeletal surgical and related procedures that require authorization from TurningPoint Healthcare Solutions LLC.**
- Prior to inpatient admission: If TurningPoint approved an authorization with an outpatient place of service and you need to change it to inpatient prior to surgery, the ordering provider should contact TurningPoint to make the change. Call TurningPoint toll-free at 1-833-217-9670 or locally at 313-908-6040.
- After inpatient admission when the setting must be inpatient based on Centers for Medicare & Medicaid Services requirements: If TurningPoint approved an outpatient setting for a procedure that’s on the CMS list of inpatient-only procedures, call TurningPoint toll-free at 1-833-217-9670 or locally at 313-908-6040. TurningPoint will update the setting on the authorization.
- Due to a change in a member’s condition during outpatient stay: If a change in a member’s condition during their outpatient stay requires an extended stay, the facility should submit an inpatient request to Blue Cross Blue Shield of Michigan or Blue Care Network. To do this, submit procedure code *99222 as outlined in the e-referral User Guide; see the “Submitting an emergency or urgent admission (includes Blue Cross member submissions)” subsection within the “Submit an inpatient authorization” section for more information. The request must meet InterQual® criteria.
Additional reminders
- When TurningPoint approves an authorization request from an ordering physician, the authorization covers both the procedure and the site of service. Facilities don’t need to submit a separate authorization request for an inpatient request if TurningPoint already approved an inpatient place of service.
- Musculoskeletal surgeries don’t require prior authorization from TurningPoint when they’re performed emergently during an inpatient admission that originated in the emergency department. For more information, see the “Do musculoskeletal procedures that are performed during an inpatient admission that originated in the emergency department require prior authorization from TurningPoint?” section in the document titled Musculoskeletal procedure authorizations: Frequently asked questions for providers.
- To update the date of service on a prior authorization, call TurningPoint.
- To update the procedure codes on an authorization after a musculoskeletal surgery has taken place, see the Postservice change request form.
- You can request additional days for an inpatient stay through the e-referral system. In the e-referral system, you’ll need to search for the member, not for the TurningPoint authorization number.
Additional information
TurningPoint Healthcare Solutions LLC is an independent company that manages authorizations for musculoskeletal surgical and related procedures for Blue Cross Blue Shield of Michigan and Blue Care Network. For more information, see the Musculoskeletal Services pages on our ereferrals.bcbsm.com website.
**See the document titled Musculoskeletal procedure codes that require authorization by TurningPoint to determine which codes require authorization. Only the codes on this list require prior authorization. Incidental codes don’t require prior authorization.
Pilot program with TurningPoint supports your patients’ musculoskeletal care
Blue Cross Blue Shield of Michigan is working with TurningPoint Healthcare Solutions LLC, an independent company, to pilot the TurningPoint Digital: Joint and Spine program.
This pilot program aims to help:
- Improve patient outcomes through early intervention before surgery and steer candidates to more conservative treatment options.
- Enhance the member experience by directing members to the next best step in their musculoskeletal care and, when needed, helping them determine where to seek care.
The program is being offered to members with Blue Cross fully insured commercial plans. Blue Cross will identify members who are candidates for this program. Then, TurningPoint will reach out to these candidates to encourage them to register for the program.
Members who engage with the program will complete an assessment through the TurningPoint Digital: Joint and Spine mobile app. Through their responses, TurningPoint will get an understanding of each member’s current treatment path or stage of treatment, which will enable TurningPoint to recommend next steps. In addition, the mobile app includes a library of physical therapy exercises that are developed by clinical experts and can help relieve members’ pain.
When a member who is engaged in this program requires a musculoskeletal procedure, less clinical review will be required because TurningPoint has already been working with the member. There is no cost to members for this program.
The pilot, which started in March 2022, will run through December 2022. At that point, we’ll determine whether the program should continue.
If you have questions about this program, send them to umproviderconcerns@bcbsm.com.
Next phase of nonclinical, transitional care program to begin May 1
The second phase of the naviHealth nonclinical, transitional care program will be available to Medicare Plus Blue℠ and BCN Advantage℠ members starting May 1.
The purpose of this next phase of the program is to reduce avoidable inpatient readmissions for members who are discharged directly to their homes from Michigan inpatient facilities. The first phase of the naviHealth program began in November 2021 and focused on supporting Medicare Advantage members discharged to certain post-acute care facilities in Southeast Michigan.
For more detailed information about the nonclinical, transitional care program, see the November 2021 Record article or the November-December 2021 BCN Provider News article.
naviHealth Inc. is an independent company that manages the nonclinical, transitional care program for Blue Cross Blue Shield of Michigan and Blue Care Network members who have Medicare Advantage plans.
Some Meijer employees or their spouses aren’t required to pay primary care and specialist office visit copays or coinsurance
Meijer employees and their spouses with Blue Cross Blue Shield of Michigan plans who successfully completed a condition management program in 2021 are eligible for waived copays or coinsurance for primary care and specialist office visits in 2022.
Because this program is enacted at the member level, the waived copayment won’t show up when you look up their benefits. To help you identify which individuals are eligible for the waived copay, we’ve mailed the eligible Meijer employees and spouses a wallet card that they can present to you at the time of service. We ask that you don’t require the patient to pay the copay up front if he or she shows you the wallet card indicating they are eligible.
If the patient is eligible, you’ll receive the copay amount when the claim is processed. Additionally, if a member who is eligible for the waived copay does pay the copay up front, the provider will be responsible for reimbursing the member.
If the patient isn’t eligible and didn’t pay the copayment up front, please bill them.
For members enrolled in health savings account plans, the deductible applies and coinsurance is waived. For members enrolled in the health reimbursement arrangement plans, the flat dollar office visit copay is waived.
Note: This applies to Meijer group number 72625. The copay waiver can apply to either a subscriber or a subscriber’s spouse, or both. Dependents aren’t eligible for the waived copay.
New HEDIS Star measure highlights importance of follow-up care after emergency department visits
Many patients with high-risk chronic conditions require important follow-up care after they’re discharged from the emergency department. Often, an emergency department discharge is based on the presumption that patients will continue necessary care with their health care providers.
The importance of continued care is highlighted in a new HEDIS® Star measure, Follow-Up After Emergency Department Visit for People with Multiple High-Risk Chronic Conditions (FMC). The measure focuses on the percentage of members who are ages 18 and older with multiple high-risk chronic conditions and who had a follow-up appointment within seven days of an emergency department visit.
Ways patients should follow up after an emergency department visit
There are several types of follow-up care that meet the criteria for FMC. Patients can follow up with a provider through an outpatient appointment by phone or virtually.
Other types of follow-up care include:
- Transitional care management
- Case management
- Complex care management
- Outpatient or telehealth visits for behavioral health
- Intensive outpatient encounter or partial hospitalization
- Community mental health center
- Electroconvulsive therapy
- Observation
Read the tip sheet on FMC for information about eligible chronic conditions, exclusions, best practices, documentation requirements and more.
HEDIS®, which stands for Healthcare Effectiveness Data Information Set, is a registered trademark of the National Committee for Quality Assurance, or NCQA.
Transitions of Care HEDIS Star measure focuses on medication management, care coordination for Medicare Advantage patients
The ineffective transferring of a patient from one care setting, such as a hospital, nursing facility, primary care provider, long-term care, home health or specialist care, to another often leads to problems, according to the American Journal of Managed Care.** It can result in confusion about treatment plans, missed follow-up appointments, patient dissatisfaction, medication nonadherence and, most importantly, unnecessary readmissions.
The Transitions of Care (TRC) HEDIS® Star measure focuses on the percentage of members who had an acute or nonacute inpatient discharge during the measurement year and who had each of the following:
- Notification of inpatient admission
- Receipt of discharge information
- Patient engagement after inpatient discharge
- Medication reconciliation post-discharge
Documentation of all four components must be in the outpatient record and accessible by the primary care provider or ongoing care provider.
If you haven’t already done so, we encourage you to establish an office practice that explains to patients why it’s crucial that they inform your office about their hospital admissions and discharges. Let them know this can improve care coordination, maintain their safety and improve their health.
Read this tip sheet to learn more about this measure, including exclusions, best practices and documentation requirements.
HEDIS®, which stands for Healthcare Effectiveness Data Information Set, is a registered trademark of the National Committee for Quality Assurance, or NCQA.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
American Heart Association offers resources to help your patients manage their health conditions
This is part of an ongoing series of articles focusing on the tools and resources available to help FEP members manage their health.
The American Heart Association has provided some easy-to-use fact sheets for people who want to better manage their health conditions. Answers by Heart** is a series of downloadable patient information sheets on various health topics.
Fact sheets include:
The fact sheets provide a place for patients to write down questions and log and track personal data, such as blood pressure, cholesterol and A1c lab results.
Blue Cross and Blue Shield Federal Employee Program® members have additional resources and tools to help with heart health as follows:
- Diabetic Meter program — 1-855-582-2024
- Members can receive one glucose meter kit per year at no cost when they meet the program criteria.
- Telehealth for nutritional counseling — 1-855-636-1579
- Members can talk with a registered dietician to evaluate nutritional needs and develop a personalized meal plan.
- Blue Cross Coordinated Care program — 1-800-775-BLUE (2583)
- This program assists members with coordination of care, education and clinical support for specific chronic and complex conditions.
- Online programs at www.fepblue.org.
- Hypertension Management program
- Members can receive a free blood pressure monitor every two years when they meet the program criteria.
- Diabetes Management program by Livongo
- Provides members who have diabetes with:
- A free glucose meter with unlimited test strips and lancets
- Personalized coaching and support
- Tobacco Cessation Incentive program***
- Members can receive tobacco cessation products at no charge after creating a Tobacco Cessation Quit Plan using the online health coach. FEP Blue Focus also has prescription drug benefits for tobacco cessation.
FEP members and health care providers who have questions about FEP support programs or benefit coverage can call Customer Service at 1-800-482-3600 or go online to www.fepblue.org.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
***Standard and Basic Option plans only.
Livongo Health Inc. is an independent company contracted by the Blue Cross and Blue Shield Service Benefit Plan to provide diabetes services to FEP members.
Virtual provider symposium: Register now for 2022 sessions
This year’s virtual provider symposiums run throughout May and June. Physicians, physician assistants, nurse practitioners, nurses and coders can receive continuing education credits for attending. You’re welcome to register for any session listed below.
We are Stars — HEDIS®/Star measure details and exclusions:
For physicians and office staff responsible for closing gaps in care related to quality adult measures. Select a date to register:
Patient Experience — Providing great service 2.0:
For physicians and office staff responsible for creating positive patient experiences. Select a date to register:
Medical record documentation and coding update:
For physicians, coders, billers and administrative staff. Select a date to register:
Questions?
If you have questions about the sessions, contact Ellen Kraft at ekraft@bcbsm.com. If you have registration questions, contact Patricia Scarlett at pscarlett@bcbsm.com.
HEDIS®, which stands for Healthcare Effectiveness Data and Information Set, is a registered trademark of the National Committee for Quality Assurance, or NCQA.
Accreditation statement: This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education through the joint providership of the Minnesota Medical Association and Blue Cross Blue Shield of Michigan. The Minnesota Medical Association is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
CME statement: The Minnesota Medical Association designates this internet live activity for a maximum of 4.5 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Lunch and learn webinars focus on risk adjustment, coding
Action item
Register now for webinars that can improve your coding processes.
Beginning in April 2022, physicians and coders can attend webinars that provide new information on documentation and coding of common and challenging diagnoses. These live lunchtime educational sessions will include an opportunity to ask questions.
Current schedule
All sessions start at noon Eastern time and generally run for 30 minutes. Click on a Register here link below to sign up.
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding registration, email Patricia Scarlett at pscarlett@bcbsm.com.

Temporary suspension of clinical review requirements for skilled nursing facility admissions has ended
The temporary suspension of clinical review requirements for admission to skilled nursing facilities for all Michigan hospitals and for hospitals in certain other states ended Feb. 28, 2022.
For SNF stays starting on or after March 1, 2022, clinical review is required on Day 1 of the stay.
The temporary suspension went into effect for Blue Cross Blue Shield of Michigan commercial, Medicare Plus Blue℠, Blue Care Network commercial and BCN Advantage℠ members as follows:
- On Sept. 1, 2021, for admissions to SNFs for hospitals in certain states. See this Provider Alert for details.
- On Sept. 20, 2021, for admissions to SNFs for hospitals in Michigan. See this Provider Alert for details.
These changes will be reflected in the following documents, which are available on our coronavirus webpage available through Provider Secured Services and on our public website at bcbsm.com/coronavirus (click on the Health Care Providers tab):
- Temporary changes due to the COVID-19 pandemic
- Ends Feb. 28, 2022: Clinical review requirements temporarily suspended for admissions to skilled nursing facilities from hospitals in certain states
In addition, the change for hospitals in certain states is reflected in the following documents for out-of-state providers, which are available from the Medical Policy & Pre-Cert/Pre-Auth Router page of our bcbsm.com website:
Outpatient facilities must bill self-administered medications through Medicare Part D
If Medicare Advantage members don’t bring their self-administered medications with them when receiving services at an outpatient facility, the facility should obtain the medications through its onsite ambulatory pharmacy, if one is available.
Facilities that obtain these medications through an onsite ambulatory pharmacy must bill them under the member’s Medicare Part D pharmacy benefits. The member is responsible for the copayment amount.
When outpatient facilities bill these medications through the member’s Medicare Part B medical benefits, the claims will be denied as not payable. To avoid these problems and help ensure that your claims are processed efficiently and correctly, bill these medications under members’ Medicare Part D pharmacy benefits.
This directive applies to Medicare Plus Blue℠ and BCN Advantage℠ members.
Reminder: Don’t resubmit claims involved in audits
Action item
Once you receive a patient listing with an audit notice letter, don’t adjust the related claims any further. Also, let your billing and finance departments know which claims we’re auditing, and ask your finance team to hold off on billing any additional audit-related claims.
Blue Cross Blue Shield of Michigan requests that you not resubmit inpatient, outpatient facility, professional or ancillary claims that are already part of an audit. Simply stated, don’t rebill to correct a mistake in billing or other errors, including ones involving a CPT procedure code, ICD-10-CM diagnosis, quantity or date of service.
Rebilling audited claims causes unnecessary disruptions for all parties.
Once you receive a patient listing with an audit notice letter:
- Don’t adjust the related claims any further.
- Let your billing and finance departments know which claims we’re auditing.
- Ask your finance team to hold billing any more audit-related claims.
Audited claims could include cases for:
- Readmissions
- Diagnosis-related group
- Catastrophic-cost outliers
- Emergency room
- High-dollar claims
- Hospital physical therapy, occupational therapy and speech language pathology
- Ambulatory surgery facilities
- Freestanding outpatient physical therapy facilities
- Home health care
Audited claims could include the following types of providers:
- M.D.s, D.O.s and specialist physicians
- Certified registered nurse anesthetists
- Durable medical equipment, prosthetics and orthotics
- Home infusion therapy
- Independent physical therapy and occupational therapy
- Ophthalmology
- Oral surgery
- Pharmacy
- Podiatry
- Provider office infusion therapy
- Urgent care
For inpatient hospitalizations where the medical record doesn’t support the inpatient setting that was billed, hospitals can rebill Medicare Part B services from the denied hospitalization and be reimbursed. To learn more, see “We’re resuming Medicare Plus Blue PPO place-of-service audits” in the October 2019 Record
Provider audit suppliers conduct audits for readmissions to the same facility occurring up to 30 days from the date the patient was released from the hospital. Hospitals are responsible for all costs pertaining to readmissions denied under the Medicare Plus Blue℠ Readmissions Reimbursement Policy.
Hospitals can no longer rebill Medicare Part B services from the denied admission and can’t combine the admissions. To learn more, see “Blue Cross implements post-pay audits related to inpatient readmissions” in the January 2021 Record.
Overweight and obesity diagnoses now payable diagnosis codes
Obesity continues to be a nationwide epidemic, affecting all age groups, according to the Centers for Disease Control and Prevention.
To help address this problem, Blue Cross Blue Shield of Michigan is updating its policy to allow overweight and obesity diagnoses to be payable diagnosis codes. This change will allow providers to better diagnose and treat obesity in their patient population, improving patients’ health and well-being. It applies to all lines of business, including Blue Cross commercial, Blue Care Network commercial and our Medicare Advantage plans (Medicare Plus Blue℠ and BCN Advantage℠).
This change doesn’t affect existing benefit structures, national coverage determinations or local coverage determinations. Click here to see the list of affected codes.
Do we have your medical records request address?
In a November 2021 Record article, we let you know that we will be working with Optum to implement some new edits. Optum is an independent company that contracts with Blue Cross Blue Shield of Michigan to identify professional and facility claims that require additional review.
One component of our relationship with Optum involves medical records requests.
To prevent claim processing delays, we need to keep your medical records request address on file. If you haven’t provided it yet or need to make changes, use the Enrollment and Change Self-Service Tool in the provider portal.
If we don’t have a medical record request address on file, the request will be sent to the mailing address. If there is no mailing address on file, the request will be sent to the billing address. If there is no billing address on file, the request will be sent to the address shown on the claim.
Outpatient PT, OT services no longer require prior authorization for Medicare Plus Blue members
For dates of service on or after April 1, 2022, Medicare Plus Blue℠ will no longer require prior authorization for outpatient physical and occupational therapy services. You can submit retroactive authorization requests to eviCore healthcare®** through July 29, 2022, for dates of service prior to April 1, 2022.
We’ll update the Medicare Plus Blue PPO Provider Manual and other documents to reflect this change.
These services will be managed by a network performance management company later this year. We’ll publish a provider alert and an article in a future issue of The Record with more details.
**eviCore healthcare is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services. For more information, go to our ereferrals.bcbsm.com website.
Clarification: Billing for IOP services provided via telemedicine for some members
We originally communicated about this in a November 2021 Record article. The information in this article is intended to clarify billing instructions for intensive outpatient program, or IOP, services provided via telemedicine.
For behavioral health IOP services provided via telemedicine with dates of service on or after Oct. 1, 2021, bill revenue code 0905 or 0906, the applicable procedure code and modifier GT or 95. We use these modifiers to identify IOP services that are delivered via telemedicine.
Follow this guidance when billing for behavioral health IOP services provided to Blue Cross commercial, BCN commercial and BCN Advantage℠ members via telemedicine. For information about billing IOP services provided to Medicare Plus Blue℠ members via telemedicine, follow guidance from the Centers for Medicare & Medicaid Services.
Keep the following in mind:
- For Blue Cross commercial members, most plans don't cover IOP services for mental health disorders. IOP services for substance use disorders must be delivered by a substance abuse treatment facility. Be sure to check member eligibility and benefits before performing services.
- Facilities can provide IOP services to BCN commercial and BCN Advantage℠ members only when their contracts specifically include IOP services.
We’ve updated the following documents to reflect this change:
- Telehealth for behavioral health providers
- Billing tips for COVID-19 at a glance
You can find these documents on our public website at bcbsm.com/coronavirus or within Provider Secured Services.
Note: For Medicare Plus Blue members, see the Medicare-covered telehealth services for the COVID-19 PHE document to determine which IOP procedures codes are billable for telemedicine.
Requirements changed for some commercial medical benefit drugs
Blue Cross Blue Shield of Michigan and Blue Care Network encourage proper utilization of high-cost medications that are covered under the medical benefit. As part of this effort, we maintain a comprehensive list of requirements for Blue Cross and BCN group and individual commercial members.
In the October 2021 through March 2022 time frame, we added either prior authorization requirements or site-of-care requirements — or both — for Blue Cross commercial and BCN commercial members for the following medical benefit drugs:
HCPCS code |
Brand name |
Generic name |
J3590** |
Susvimo® |
ranibizumab injection, for ocular implant |
J3590** |
Cortrophin™ |
corticotrophin |
J3590** |
Vyvgart™ |
efgartigimod alfa-fcab |
J3590** |
Leqvio® |
inclisiran |
J3590** |
Tezspire™ |
tezepelumab-ekko |
J3590** |
Vabysmo™ |
faricimab-svoa |
**Will become a unique code
See the Blue Cross and BCN utilization management medical drug list for additional details. This list is available on the following pages of the ereferrals.bcbsm.com website:
As a reminder, an authorization approval isn’t a guarantee of payment. Health care providers need to verify eligibility and benefits for members.
Additional information
For Blue Cross commercial groups, this authorization requirement applies only to groups that currently participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. A link to this list is also available on the Blue Cross Medical Benefit Drugs page of the ereferrals.bcbsm.com website.
Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don't participate in the standard prior authorization program.
Enjaymo, Vabysmo and Byooviz to require prior authorization for Medicare Advantage members
Providers must submit prior authorization requests through the NovoLogix® online tool for the following drugs covered under the medical benefit for our Medicare Advantage members:
- For dates of service on or after March 7, 2022:
- Enjaymo™ (sutimlimab-jome), HCPCS code J3590
- Vabysmo™ (faricimab-svoa), HCPCS code J3590
- For dates of service on or after June 6, 2022:
- Byooviz® (ranibizumab-nuna), HCPCS code J3590
This requirement applies to Medicare Plus Blue℠ and BCN Advantage℠ members.
When prior authorization is required
We require prior authorization when these drugs are administered in any site of care other than inpatient hospital (place of service code 21) and are billed as follows:
- Electronically through an 837P transaction or on a professional CMS-1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
Submitting prior-authorization requests
Submit prior authorization requests for these drugs through NovoLogix. It offers real-time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to NovoLogix.
If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
We'll update the list to reflect these changes prior to the effective date.
Nyvepria, Rybrevant to require prior authorization for URMBT members with Blue Cross non-Medicare plans
For dates of service on or after May 16, 2022, we're adding prior authorization requirements for the following drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans:
- Nyvepria™ (pegfilgrastim-apgf), HCPCS code Q5122
- Rybrevant™ (amivantamab-vmjw), HCPCS code J9061
Submit prior authorization requests through AIM Specialty Health®.
Notes:
- The prior authorization requirement applies when these drugs are administered in an outpatient setting.
- This requirement doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714).
How to submit authorization requests
Submit prior authorization requests to AIM using one of the following methods:
More about the authorization requirements
Authorization isn't a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, see:
We’ll update the pertinent drug lists to reflect the information in this message prior to the effective date.
Accredo manages prior authorization requests for other medical benefit drugs.
AIM is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to provide benefit management services.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
We’ve updated payment policy for Unna boot or multilayer compression dressing
Blue Cross Blue Shield of Michigan has updated its payment policy to align with the Centers for Medicare & Medicaid Services’ National Correct Coding Initiative guidelines for the application of Unna boot or multilayer compression dressing when reported with a wound debridement procedure.
CPT codes *29580 (Unna boot) and *29581 (application of multilayer compression system) aren’t separately payable when applied at the same site or extremity where CPT codes *11042-*11047 or *97597-*97598 wound debridement is performed. This payment policy aligns with NCCI procedure-to-procedure coding edits and statements contained in the NCCI Policy Manual for Medicare Services.
TurningPoint authorizations for musculoskeletal surgical and related procedures are valid for 6 months
Prior authorization requests approved by TurningPoint Healthcare Solutions LLC on or after Jan. 1, 2022, are valid for six months from the planned date of service for all outpatient musculoskeletal procedures, including pain management procedures.
For example, if TurningPoint approves an authorization request for a service planned for June 1, the approval will be valid from June 1 through Nov. 30. If the surgery is performed during that time period, the authorization will match the claim without any changes to the authorization.
We updated the following documents to reflect this change:
Note: Prior authorization requests that were approved on or before Dec. 31, 2021, are valid for 30 days from the planned date of service.
TurningPoint Healthcare Solutions LLC is an independent company that manages authorizations for musculoskeletal surgical and related procedures for Blue Cross Blue Shield of Michigan and Blue Care Network.
Musculoskeletal procedure authorizations: Reminders for facilities
This article provides a review of how to change the place of service from outpatient to inpatient for musculoskeletal surgical and related procedures that require authorization from TurningPoint Healthcare Solutions LLC.**
- Prior to inpatient admission: If TurningPoint approved an authorization with an outpatient place of service and you need to change it to inpatient prior to surgery, the ordering provider should contact TurningPoint to make the change. Call TurningPoint toll-free at 1-833-217-9670 or locally at 313-908-6040.
- After inpatient admission when the setting must be inpatient based on Centers for Medicare & Medicaid Services requirements: If TurningPoint approved an outpatient setting for a procedure that’s on the CMS list of inpatient-only procedures, call TurningPoint toll-free at 1-833-217-9670 or locally at 313-908-6040. TurningPoint will update the setting on the authorization.
- Due to a change in a member’s condition during outpatient stay: If a change in a member’s condition during their outpatient stay requires an extended stay, the facility should submit an inpatient request to Blue Cross Blue Shield of Michigan or Blue Care Network. To do this, submit procedure code *99222 as outlined in the e-referral User Guide; see the “Submitting an emergency or urgent admission (includes Blue Cross member submissions)” subsection within the “Submit an inpatient authorization” section for more information. The request must meet InterQual® criteria.
Additional reminders
- When TurningPoint approves an authorization request from an ordering physician, the authorization covers both the procedure and the site of service. Facilities don’t need to submit a separate authorization request for an inpatient request if TurningPoint already approved an inpatient place of service.
- Musculoskeletal surgeries don’t require prior authorization from TurningPoint when they’re performed emergently during an inpatient admission that originated in the emergency department. For more information, see the “Do musculoskeletal procedures that are performed during an inpatient admission that originated in the emergency department require prior authorization from TurningPoint?” section in the document titled Musculoskeletal procedure authorizations: Frequently asked questions for providers.
- To update the date of service on a prior authorization, call TurningPoint.
- To update the procedure codes on an authorization after a musculoskeletal surgery has taken place, see the Postservice change request form.
- You can request additional days for an inpatient stay through the e-referral system. In the e-referral system, you’ll need to search for the member, not for the TurningPoint authorization number.
Additional information
TurningPoint Healthcare Solutions LLC is an independent company that manages authorizations for musculoskeletal surgical and related procedures for Blue Cross Blue Shield of Michigan and Blue Care Network. For more information, see the Musculoskeletal Services pages on our ereferrals.bcbsm.com website.
**See the document titled Musculoskeletal procedure codes that require authorization by TurningPoint to determine which codes require authorization. Only the codes on this list require prior authorization. Incidental codes don’t require prior authorization.
Lunch and learn webinars focus on risk adjustment, coding
Action item
Register now for webinars that can improve your coding processes.
Beginning in April 2022, physicians and coders can attend webinars that provide new information on documentation and coding of common and challenging diagnoses. These live lunchtime educational sessions will include an opportunity to ask questions.
Current schedule
All sessions start at noon Eastern time and generally run for 30 minutes. Click on a Register here link below to sign up.
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding registration, email Patricia Scarlett at pscarlett@bcbsm.com.

Outpatient facilities must bill self-administered medications through Medicare Part D
If Medicare Advantage members don’t bring their self-administered medications with them when receiving services at an outpatient facility, the facility should obtain the medications through its onsite ambulatory pharmacy, if one is available.
Facilities that obtain these medications through an onsite ambulatory pharmacy must bill them under the member’s Medicare Part D pharmacy benefits. The member is responsible for the copayment amount.
When outpatient facilities bill these medications through the member’s Medicare Part B medical benefits, the claims will be denied as not payable. To avoid these problems and help ensure that your claims are processed efficiently and correctly, bill these medications under members’ Medicare Part D pharmacy benefits.
This directive applies to Medicare Plus Blue℠ and BCN Advantage℠ members.
Requirements changed for some commercial medical benefit drugs
Blue Cross Blue Shield of Michigan and Blue Care Network encourage proper utilization of high-cost medications that are covered under the medical benefit. As part of this effort, we maintain a comprehensive list of requirements for Blue Cross and BCN group and individual commercial members.
In the October 2021 through March 2022 time frame, we added either prior authorization requirements or site-of-care requirements — or both — for Blue Cross commercial and BCN commercial members for the following medical benefit drugs:
HCPCS code |
Brand name |
Generic name |
J3590** |
Susvimo® |
ranibizumab injection, for ocular implant |
J3590** |
Cortrophin™ |
corticotrophin |
J3590** |
Vyvgart™ |
efgartigimod alfa-fcab |
J3590** |
Leqvio® |
inclisiran |
J3590** |
Tezspire™ |
tezepelumab-ekko |
J3590** |
Vabysmo™ |
faricimab-svoa |
**Will become a unique code
See the Blue Cross and BCN utilization management medical drug list for additional details. This list is available on the following pages of the ereferrals.bcbsm.com website:
As a reminder, an authorization approval isn’t a guarantee of payment. Health care providers need to verify eligibility and benefits for members.
Additional information
For Blue Cross commercial groups, this authorization requirement applies only to groups that currently participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. A link to this list is also available on the Blue Cross Medical Benefit Drugs page of the ereferrals.bcbsm.com website.
Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don't participate in the standard prior authorization program.
Enjaymo, Vabysmo and Byooviz to require prior authorization for Medicare Advantage members
Providers must submit prior authorization requests through the NovoLogix® online tool for the following drugs covered under the medical benefit for our Medicare Advantage members:
- For dates of service on or after March 7, 2022:
- Enjaymo™ (sutimlimab-jome), HCPCS code J3590
- Vabysmo™ (faricimab-svoa), HCPCS code J3590
- For dates of service on or after June 6, 2022:
- Byooviz® (ranibizumab-nuna), HCPCS code J3590
This requirement applies to Medicare Plus Blue℠ and BCN Advantage℠ members.
When prior authorization is required
We require prior authorization when these drugs are administered in any site of care other than inpatient hospital (place of service code 21) and are billed as follows:
- Electronically through an 837P transaction or on a professional CMS-1500 claim form
- Electronically through an 837I transaction or by using the UB04 claim form for a hospital outpatient type of bill 013x
Submitting prior-authorization requests
Submit prior authorization requests for these drugs through NovoLogix. It offers real-time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services, you already have access to NovoLogix.
If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form and fax it to the number on the form.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
We'll update the list to reflect these changes prior to the effective date.
Nyvepria, Rybrevant to require prior authorization for URMBT members with Blue Cross non-Medicare plans
For dates of service on or after May 16, 2022, we're adding prior authorization requirements for the following drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross Blue Shield of Michigan non-Medicare plans:
- Nyvepria™ (pegfilgrastim-apgf), HCPCS code Q5122
- Rybrevant™ (amivantamab-vmjw), HCPCS code J9061
Submit prior authorization requests through AIM Specialty Health®.
Notes:
- The prior authorization requirement applies when these drugs are administered in an outpatient setting.
- This requirement doesn’t apply to the UAW Retiree Health Care Trust (group number 70605) or the UAW International Union (group number 71714).
How to submit authorization requests
Submit prior authorization requests to AIM using one of the following methods:
More about the authorization requirements
Authorization isn't a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit for UAW Retiree Medical Benefits Trust members with Blue Cross non-Medicare plans, see:
We’ll update the pertinent drug lists to reflect the information in this message prior to the effective date.
Accredo manages prior authorization requests for other medical benefit drugs.
AIM is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to provide benefit management services.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Next phase of nonclinical, transitional care program to begin May 1
The second phase of the naviHealth nonclinical, transitional care program will be available to Medicare Plus Blue℠ and BCN Advantage℠ members starting May 1.
The purpose of this next phase of the program is to reduce avoidable inpatient readmissions for members who are discharged directly to their homes from Michigan inpatient facilities. The first phase of the naviHealth program began in November 2021 and focused on supporting Medicare Advantage members discharged to certain post-acute care facilities in Southeast Michigan.
For more detailed information about the nonclinical, transitional care program, see the November 2021 Record article or the November-December 2021 BCN Provider News article.
naviHealth Inc. is an independent company that manages the nonclinical, transitional care program for Blue Cross Blue Shield of Michigan and Blue Care Network members who have Medicare Advantage plans.

Orthotic Devices Policy updated to cover K1015, effective Nov. 1, 2021
Effective Nov. 1, 2021, an adjustable corrective foam foot brace with a rigid heel cup, HCPCS code K1015, may be used on pediatric patients. It’s used for the treatment of metatarsus adductus.
For the full policy, see the April billing chart.
|



