|
May 2019

How to request retroactive prior authorization for commercial PPO Radiology Management Program
Providers have up to 90 days past the date of service to request retro prior authorization through AIM Specialty HealthSM for Blue Cross Blue Shield of Michigan commercial PPO members participating in our Radiology Management Program. Although 90 days is allowed, we encourage providers to obtain prior authorization before administering services.
Prior authorization requests are initiated through the AIM online portal and by phone. AIM completes approximately 90 percent of authorizations within 48 hours of the request.
How to submit a request
- Retro prior authorization requests
- Only by phone at 1-800-728-8008
- All other requests
We’ll consider requests older than 90 days but less than 180 days from the date of service for a coordination of benefits decision. Documentation of the other carriers’ remittance must be submitted. For these considerations, call our provider service phone numbers:
- Professional at 1-800-344-8525
- Provider Inquiry — Facility at 1-800-249-5103
Use your company email for Provider Secured Services access requests
We’re always looking for additional ways to protect our member’s information and keep your account secure. That’s why we’d like to connect your online account to an email address that’s related to your business rather than a public email provider such as Hotmail, Gmail or Yahoo.
If you have a company email address, please include it on your request for access or changes to your Provider Secured Services account at bcbsm.com. If you’re not sure whether a company email address is available to you, check with your website administrator.
Most websites offer a domain email free with your account. If you’re a smaller practice that doesn’t host a website, we’ll accept your request with the email you use to conduct your business.
May is Mental Health Month
May is Mental Health Month, a good time to remind your patients of the important role that good mental health plays in overall wellness.
New Directions Behavioral Health, an independent company that administers behavioral health services for many of our Blue Cross Blue Shield of Michigan members, has compiled a wide array of educational materials, posters, stories and tips to help promote good mental health.
Topics include:
- Mental health and substance use disorders
- Warning signs of mental illness
- What to say to someone struggling with mental health
- Tips for improving mental health
We encourage you to check out our Engage page to learn more. Simply scroll down to the Mental Health Awareness section of the page.
For more information
- Go to the Mind section of A Healthier Michigan at ahealthiermichigan.org/mind for blogs highlighting mental health issues and the mind-body connection.
- Check out the mental health-related podcasts on A Healthier Michigan to hear experts from Blue Cross discuss topics such as stress, anxiety and relaxation.
- Follow Blue Cross on Facebook and Twitter for up-to-date mental health information.
Battling the opioid epidemic: A roundup of news and information
CMS develops Medicare Part D opioid mapping tool
The Centers for Medicare & Medicaid Services has developed a Medicare Part D opioid prescribing mapping tool. This interactive tool shows geographic comparisons at the state, county and ZIP code levels of de-identified Medicare Part D opioid prescriptions filled within the U.S. For more information, click here.
Study explores geographic variation in opioid prescribing
How have key opioid prescription measures changed by state between 2006 and 2017 in the U.S.? A new study, published in JAMA, examines this question. To read more, click here.
Bloomberg gives Michigan $10 million to fight opioid crisis
Former New York Mayor Michael Bloomberg traveled to Michigan in March to announce a $10 million contribution to the state’s efforts to fight the opioid crisis, the Detroit Free Press reported. The money will come from Bloomberg Philanthropies, which will partner with up to 10 states over the next three years and invest $50 million to support state programs to develop treatment and prevention programs. To read more, click here.
In a related item in The Detroit News, Bloomberg and Michigan Gov. Gretchen Whitmer wrote an opinion piece on what state governments have been doing and Michigan’s fight to combat the opioid crisis.
New law makes it easier to refuse opioids
Michigan has enacted a law that allows people to refuse opioid medications by placing a non-opioid advance directive in their medical file, the Detroit Free Press reported March 28. For details, click here.
Michigan schools stocking first aid kits with Narcan®
A number of Detroit-area schools are stocking their facilities with Narcan, the overdose-reversing drug, and training school staff to use it, the Detroit Free Press reported March 14. To read more, click here.
Billing chart: Blues highlight medical, benefit policy changes
You’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart.
This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures.
You will also see that descriptions for the codes are no longer included. This is a result of recent negotiations with the AMA on use of the codes.
We will publish information about new BCBS groups or changes to group benefits under the Group Benefit Changes heading.
For more detailed descriptions of the BCBSM policies for these procedures, please check under the Medical/Payment Policy tab in Explainer on web-DENIS. To access this online information:
- Log in to web-DENIS.
- Click on BCBSM Provider Publications & Resources.
- Click on Benefit Policy for a Code.
- Click on Topic.
- Under Topic Criteria, click on the drop-down arrow next to Choose Identifier Type and then click on HCPCS Code.
- Enter the procedure code.
- Click on Finish.
- Click on Search.
| Code* |
BCBSM changes to:
Basic Benefit and Medical Policy, Group
Variations Payment Policy, Guidelines
|
| UPDATES TO PAYABLE PROCEDURES |
Established codes:
19303, 19304, 19318, 19350, 54520, 55970, 55980, 56805, 57291, 57292, 57335, 58150, 58152, 58180, 58260, 58262, 58275, 58291, 58541, 58542, 58543, 58544, 58550, 58552, 58553, 58554
Note: Code 17380 may be considered established when performed to prepare tissues before genital surgery when billed with 55970, 55980, 57291, 57292 or 57335 — see inclusions.
Investigational, not medically necessary, etc.:
11950, 11951, 11952, 11954,15820, 15821, 15822, 15823, 15824,15825, 15826, 15828, 15830, 15832,15833, 15834, 15835, 15836, 15837,15838, 15839, 15876, 15877, 15878, 15879, 17380, 21120, 21121, 21122, 21123
21125, 21127, 30400, 30410, 30420, 30430, 30435, 30450
|
Basic benefit and medical policy
Transgender services
The criteria have been updated for the transgender services policy. This policy is effective May 1, 2019.
Assessment, diagnosis and treatment should be provided through a multidisciplinary gender services clinic or program affiliated with a major medical center. If this level of service is unavailable, there should be documentation that reflects a coordinated approach to care by specialists involved (mental health specialists, physicians, surgeons, etc.).
Puberty suppression**
Puberty suppression hormones for adolescents may be indicated for members who meet all the following inclusionary criteria:
- Onset of puberty to at least Tanner Stage 2.
- The adolescent has demonstrated a long-lasting and intense pattern of gender nonconformity or gender dysphoria (whether suppressed or expressed).
- Gender dysphoria emerged or worsened with the onset of puberty.
- Any coexisting psychological, medical or social problems that could interfere with treatment (e.g., that may compromise treatment adherence) have been addressed, such that the adolescent’s situation and functioning are stable enough to start treatment.
- The adolescent has given informed consent and, particularly when the adolescent hasn’t reached the age of medical consent, the parents or other legally authorized caretakers or guardians have consented to the treatment and are involved in supporting the adolescent throughout the treatment process.
- The absence of contraindications to therapy in the judgment of the managing physician.
**Medications for puberty suppression may be managed under the member’s pharmacy benefit.
Hormone therapy**
Hormone therapy may be indicated for members who meet all the following inclusionary criteria:
- Persistent, well-documented gender dysphoria
- Capacity to make a fully informed decision and to consent for treatments
- Eighteen years of age or older (age of majority)
- If significant medical or mental health concerns are present, they must be reasonably well-controlled.
- The absence of contraindications to therapy in the judgment of the managing physician
**Medications for hormone therapy may be managed under the member’s pharmacy benefit.
Gender reassignment surgery
Gender reassignment surgery may be indicated for members who meet all the following inclusionary criteria:
- Persistent, well-documented gender dysphoria
- The provider must supply documentation that supports the member meets criteria for gender reassignment surgery. This includes detailed psychological assessments by two mental health providers:
- Psychiatrist
- Ph.D. prepared clinical psychologist
- Master’s level clinicians who are licensed to practice independently in their state
- Eighteen years of age or older
- Capacity to make a fully informed decision and to consent for treatment
- If significant medical or mental health concerns are present, they must be controlled.
- Twelve continuous months of hormone therapy** as appropriate to the patient’s gender role (unless there is a contraindication to hormonal therapy):
- **Hormonal therapy is not required before a mastectomy in biological female-to-male patients.
- The aim of hormone therapy before gonadectomy is primarily to introduce a period of reversible estrogen or testosterone suppression, before the patient undergoes irreversible surgical intervention.
- Twelve continuous months of living in a gender role that is congruent with their gender identity:
- Living in a gender role congruent with gender identity for 12 continuous months is not required before a mastectomy in biological female-to-male patients.
Electrolysis
- If gender reassignment surgery is approved for a biological male transitioning to female, permanent hair removal (by electrolysis) may be considered established following medical review. Permanent hair removal is considered established only when the scrotal and surrounding tissues are used in the surgical construction of the vagina.
- If gender reassignment surgery is approved for a biological female transitioning to male, permanent hair removal (by electrolysis) may be considered established following medical review. Permanent hair removal is considered established only when free flap or other donor tissues are used for phalloplasty that’s performed in conjunction with vaginectomy and full-length urethroplasty.
Some patients receiving transgender services may require and benefit from ongoing behavioral health services, including psychotherapy.
Exclusions:
- Transgender services aren’t covered if contract or certificate language contains specific exclusion of these services.
- Reversal of transgender surgical procedures.
- All surgical procedures that are primarily cosmetic and not medically necessary, including but not limited to:
- Abdominoplasty
- Blepharoplasty
- Breast enhancements
- Brow lift
- Calf implants
- Cheek/malar implants
- Chin/nose implants
- Chondrolaryngoplasty (Adam’s apple reduction)
- Collagen injections
- Drugs for hair loss or growth
- Forehead lift
- Hair removal (for exception: see inclusions, electrolysis)
- Hair transplantation
- Lip reduction
- Liposuction
- Mastopexy
- Neck tightening
- Pectoral implants
- Removal of redundant skin
- Rhinoplasty
- Speech-language therapy
- Non-covered services
|
81235, 81275, 81404,** 81405,** 81406,** 81479**
Revenue code 0310
**Codes require individual consideration.
|
Basic benefit and medical policy
Genetic testing: Molecular analysis for targeted therapy of non-small cell lung cancer
The medical policy statement has been updated to reflect changes to coverage and criteria, effective March 1, 2019.
EGFR gene
The safety and effectiveness of analysis of somatic variants in exons 18 (such as G719X), 19 (such as L858R, T790M), 20 (such as S678I) or 21 (such as L861Q) within the EGFR gene have been established to predict treatment response to an EGFR tyrosine kinase inhibitor (TKI) therapy (e.g., erlotinib [Tarceva®], gefitinib [Iressa®] or afatinib [Gilotrif®]), or osimertinib (Tagrisso) in patients with advanced lung adenocarcinoma or advanced squamous cell NSCLC.
The analysis for other EGFR mutations within exons 22-24, or other applications related to NSCLC, is considered experimental. The peer-reviewed medical literature hasn’t yet demonstrated the clinical utility of this testing for this indication.
ALK gene
The safety and effectiveness of analysis of somatic rearrangement mutations of the ALK gene have been established. It’s an effective diagnostic option for predicting treatment response to crizotinib (Xalkori®) or ceritinib (Zykadia™) in patients with advanced lung adenocarcinoma and large cell carcinoma or for patients in whom an adenocarcinoma component can’t be excluded.
Analysis of somatic rearrangement mutations of the ALK gene is considered experimental in all other situations.
BRAF V600E gene
Analysis of the BRAF V600E variant is established to predict treatment response to BRAF or MEK inhibitor therapy (e.g., dabrafenib [Tafinlar] and trametinib [Mekinist®]), in patients with advanced lung adenocarcinoma or in whom an adenocarcinoma component can’t be excluded.
ROS1 gene
Analysis of somatic rearrangement variants of the ROS1 gene is established to predict treatment response to ALK inhibitor therapy (crizotinib [Xalkori]) in patients with advanced lung adenocarcinoma or in whom an adenocarcinoma component can’t be excluded.
KRAS gene
Analysis of somatic mutations of the KRAS gene is established as a technique to predict treatment nonresponse to anti-EGFR therapy with tyrosine kinase inhibitors and for the use of the anti-EGFR monoclonal antibody cetuximab in NSCLC. The peer-reviewed medical literature has demonstrated the clinical utility of this testing for this indication.
Other genes
Analysis for genetic alterations in the genes RET, MET, and HER2 for targeted therapy in patients with NSCLC, is considered experimental. The peer-reviewed medical literature hasn’t yet demonstrated the clinical utility of this testing for this indication.
Payment policy
Additional payable diagnoses have been added:
C33, C34.00-C34.02, C34.10-C34.12, C34.2, C34.30-C34.32, C34.80-C34.82, C34.90-C34.92
|
| POLICY CLARIFICATIONS |
J9351
|
Basic benefit and medical policy
Hycamtin (topotecan) Effective Sept. 5, 2018, Hycamtin (topotecan) is covered for the following updated FDA-approved indications:
Hycamtin (topotecan) for injection is a topoisomerase inhibitor indicated for treatment of:
- Patients with metastatic ovarian cancer after disease progression on or after initial or subsequent chemotherapy, as a single agent
- Patients with small cell lung cancer platinum-sensitive disease who progressed at least 60 days after initiation of first-line chemotherapy, as a single agent
- Patients with Stage IV-B, recurrent or persistent cervical cancer that isn’t amenable to curative treatment, in combination with cisplatin
Dosing information:
- Ovarian cancer and small cell lung cancer: 1.5 mg/m2 by intravenous infusion over 30 minutes daily for five consecutive days, starting on Day 1 of a 21-day cycle
- Cervical cancer: 0.75 mg/m2 by intravenous infusion over 30 minutes on Days 1, 2 and 3, with cisplatin 50 mg/m2 on Day 1 of a 21-day cycle
- Renal impairment: Reduce dose if creatinine clearance (CLcr) 20 to 39 mL/min
Pharmacy doesn’t require preauthorization of this drug.
NDC: 00078-0674-61
|
| EXPERIMENTAL PROCEDURES |
0493T
|
Basic benefit and medical policy
Near infrared spectroscopy for wound examination The use of a near infrared spectroscopic device to examine wounds is considered experimental. There is insufficient evidence of its effectiveness on health outcomes.
This policy is effective May 1, 2019.
|
81479, 81599
|
Basic benefit and medical policy
Gene genetic testing using single nucleotide variants to predict risk of nonfamilial breast cancer Regarding the policy titled Genetic Testing (Single Nucleotide Variants) To Predict Risk of Nonfamiliar Breast Center, the effectiveness and clinical utility of genetic testing using single nucleotide variants to predict future risk of breast cancer is considered experimental. There is insufficient scientific evidence on the analytical and clinical validity as well as clinical utility of these tests on patient management and outcomes.
This policy is effective March 1, 2019.
|
81541, 81551, 81599
|
Basic benefit and medical policy
Gene expression profile analysis for risk stratification for prostate cancer management Gene expression analysis to guide management of prostate cancer continues to be experimental in all situations. There is insufficient evidence in the peer-reviewed medical literature to establish the analytic validity, clinical validity or clinical utility of this testing.
This policy is effective May 1, 2019.
|
83698, 0423T
|
Basic benefit and medical policy
Fluad measurement of Lp-PLA2 and sPLA-IIA in cardiovascular risk The measurement of lipoprotein-associated Phospholipase A2 (Lp-PLA2) in the assessment of cardiovascular risk is considered experimental. While this service may be safe, its usefulness in the clinical management of atherosclerosis hasn’t been established.
Measurement of secretory type II Phospholipase A2 (sPLA2-IIA) to determine risk of cardiovascular disease is considered experimental. Current medical literature doesn’t support a causal relationship between sPLA2-IIA and cardiovascular disease.
This policy has been updated, effective May 1, 2019.
|
90689
|
Basic benefit and medical policy
Fluad Quadrivalent Pediatric vaccine The Fluad Quadrivalent Pediatric™ vaccine is considered experimental. The U.S. Food and Drug Administration hasn’t approved this vaccine and it’s not currently recommended by the Advisory Committee on Immunization Practices.
This policy is effective Jan. 1, 2019.
|
B4105
|
Basic benefit and medical policy
Relizorb Relizorb® is considered experimental. There is insufficient scientific evidence to indicate that this technology is beneficial.
This policy is effective May 1, 2019.
|
Note: The Federal Employee Program® follows its own criteria related to procedure codes J3490, J3590, J9271 and J9305.

Reminder: Prior authorization changes to AIM authorization program for MA PPO members begin May 1, 2019
Starting May 1, 2019, certain cardiac procedures and in-lab sleep testing will require prior authorization through AIM Specialty Health for Medicare Plus BlueSM PPO members. This includes UAW Retiree Medical Benefits Trust members with Medicare Plus Blue coverage.
The PPO radiology management program added the cardiology and in-lab sleep study prior authorization program earlier this year.
The additional cardiac procedures below require prior authorization for Medicare Plus Blue members:
Percutaneous coronary intervention
- *92920
- *92924
- *92928
- *92933
- *92937
- *92943
Diagnostic coronary catheterization
Note: URMBT commercial PPO members are excluded from the prior authorization requirement for the expanded cardiology services described above.
Blue Cross Blue Shield of Michigan now requires prior authorization for in-lab sleep testing by in-state providers for Medicare Plus Blue for the following procedure codes:
- *95805
- *95807
- *95808
- *95810
- *95811
In addition, the high-tech radiology program (breast MRI) now requires Blue Cross commercial PPO and Medicare Plus Blue PPO members to get prior authorization through AIM for the procedure codes below:
How to request authorization
For more information
Refer to the February 2019 Record article or the FAQ for more information.
Medical drug prior authorization program expanding
Starting July 1, 2019, Khapzory™ and Fusilev® will be added to the Medical Drug Prior Authorization Program for Blue Cross Blue Shield of Michigan PPO commercial members.
- Khapzory (levoleucovorin sodium, HCPCS code J3490)
- Fusilev (levoleucovorin calcium, HCPCS code J0641)
This change applies to members starting therapy on or after July 1. There is nothing needed for members currently on Fusilev or Khapzory. These drugs are already included in the prior authorization program for Blue Care Network HMOSM commercial members.
The authorization requirement only applies to groups currently participating in the commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To find a list of groups that don’t take part in the program, follow these steps:
- Log in to Provider Secured Services.
- Click on BCBSM Provider Publications and Resources.
- Click on Newsletters & Resources.
- Click on Forms.
- Click on Physician administered medications.
- Click on BCBSM Medical Drug Prior Authorization Program list of groups that have opted out.
These changes don’t apply to BCN AdvantageSM, Blue Cross Medicare Plus BlueSM PPO or Federal Employee Program® members.
A prior authorization approval isn’t a guarantee of payment. Providers need to verify eligibility and benefits for members. Members are responsible for the full cost of medications not covered under their medical benefit coverage.
For a list of requirements for drugs covered under the medical benefit and for directions on how to submit a prior authorization request, see the following resources:
The new prior authorization requirement for Khapzory and Fusilev will be reflected in the medical drug list before the July 1 effective date.
Clarification on reimbursement of CPT code *99354
The American Medical Association updated the nomenclature for CPT code *99354 to include or psychotherapy. This code remains classified as an evaluation and management code. Therefore, providers who are ineligible to perform and bill E&M codes can’t bill code *99354 as an add-on to other codes that are payable.
If ineligible providers bill this code, it will reject as not payable for your specialty and provider liability will apply.
CAQH Direct Assure 2.0 phases III and IV continue to roll out
We’re continuing to roll out CAQH Direct Assure. Phase III was rolled out at the end of March for our behavioral health providers. This is an effort to assist these providers in managing their demographic data and help ensure the data accuracy of behavioral health practitioners displayed in the directory.
We’ll roll out phases IV-VI in the last month of each quarter of 2019.
We’re scheduled to roll out phase IV at the end of June to specialists who are likely to be audited by the Centers for Medicare & Medicaid Services:
- Primary care physicians
- Cardiologists
- Oncologists
- Ophthalmologists
Background
CAQH Direct Assure allows you to see specific group affiliation information that’s in our system so you can make updates and add group information to an individual provider’s CAQH record. Direct Assure also allows certain group changes made in CAQH to be updated in the Blue Cross Blue Shield of Michigan system so you no longer have to make updates in both areas — the CAQH and Blue Cross systems.
We decided to do a phased rollout to small subsets of providers. We rolled out the first phase to 4 percent of our practitioners in June 2018 and the second phase to an additional 11 percent in January 2019.
Progress to date
The providers who participated in the first phase had higher-than-average accuracy scores for demographic data and better alignment of information between our data and CAQH data. We continue to work closely with provider organizations and groups that have participating providers to obtain feedback, educate them on how to manage their data in Direct Assure and provide updates on the overall project.
If you have questions, reach out to your provider consultant or contact Provider Enrollment at 1‑800‑822‑2761.
Reminder: New approach aims to educate, promote appropriate use of evaluation and management codes
Selecting the CPT code that best reflects the complexity of an evaluation and management service is a big challenge for coders. Perhaps that’s why there are more mistakes made with E&M coding than with other services.
To help health care providers and their office staff determine which E&M code appropriately reflects the complexity of a visit, Blue Cross Blue Shield of Michigan has contracted with Change Healthcare to implement its Coding Advisor solution. Change Healthcare reviews the E&M codes billed and other scenarios, such as modifier 25, observation care and nursing facility care, on claims submitted to Blue Cross. The program provides useful data insights to the provider community and works to maximize coding efficiency and accuracy through up-front education, rather than a traditional post-claim review process.
On March 18, 2019, Change Healthcare began to reach out by letter or phone to select providers who submit claims to Blue Cross with E&M codes. Coding Advisor will compare the billing of those codes across provider types with their peers through a physician profile. See example of a physician profile below.
Throughout the course of this program, Coding Advisor will continue to monitor billing practices and send updated reports periodically. It may contact your practice to discuss coding variances and to offer one-on-one coding education. All correspondence will be sent to you from Change Healthcare.
If you have any questions, call Change Healthcare Customer Support at 1-844-592-7009, Option 3.
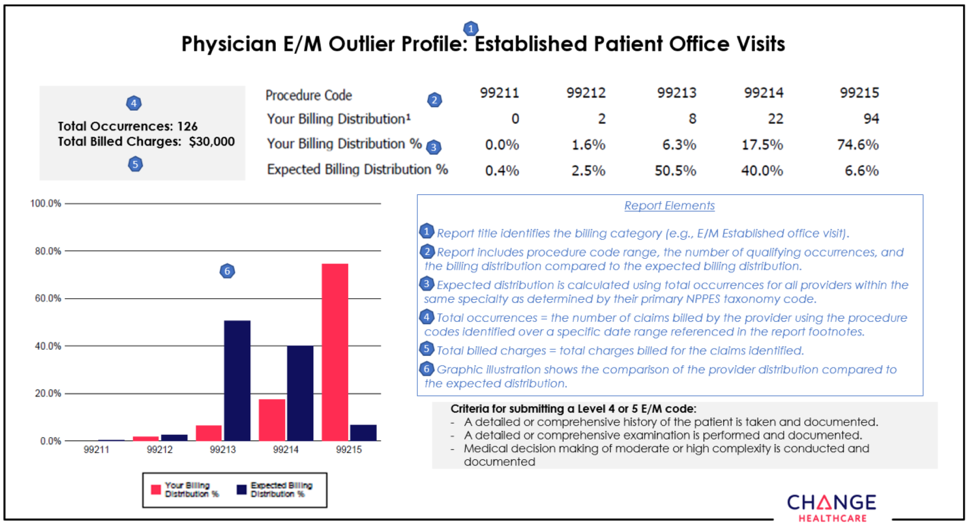
None of the information included in this article is intended to be legal advice and, as such, it remains the health care provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
Tips for billing medical drugs correctly
Medical billers often question how to bill appropriate quantities of National Drug Codes under the medical drug benefit. To ensure the payment you receive is accurate, submit the appropriate quantities for all NDCs.
| Procedure code |
Billable units** |
Example dose |
HCPCS quantity (for dose example) |
NDC |
NDC billable unit** |
Correct NDC quantity (for dose example) |
| 90633 |
N/A |
0.5 ml |
1 |
00006483141 |
ml |
ML0.5 |
| 90647 |
N/A |
0.5 ml |
1 |
00006489700 |
ml |
ML0.5 |
| 90651 |
N/A |
0.5 ml |
1 |
00006411903 |
ml |
ML0.5 |
| 90670 |
0.5 ml |
0.5 ml |
1 |
00005197102 |
ml |
ML0.5 |
| 90686 |
0.5 ml |
0.5 ml |
1 |
49281041850 |
ml |
ML0.5 |
| 90715 |
0.5 ml |
0.5 ml |
1 |
58160084252 |
ml |
ML0.5 |
| 90734 |
N/A |
1 dose |
1 |
49281058905 |
If powder or oral tablet, use UN. If liquid in a vial, use ML. |
ML0.5 |
| 90744 |
1 dose |
0.5 ml |
1 |
58160082052 |
ml |
ML0.5 |
| J0585 |
1 unit |
100 units |
100 |
00023114501 |
UN |
UN1 |
| J0641 |
0.5 mg |
500 mg |
1000 |
00781320194 |
If powder or oral tablet, use UN. If liquid in a vial, use ML. |
ML50 |
| J0696 |
250 mg |
250 mg |
1 |
00409733701 |
If powder or oral tablet, use UN. If liquid in a vial, use ML. |
UN1 |
| J1030 |
40 mg |
40 mg |
1 |
00009028003 |
ml |
ML1 |
| J1040 |
80 mg |
80 mg |
1 |
00009030602 |
ml |
ML1 |
| J1100 |
1 mg |
1 mg |
1 |
63323016505 |
ml |
ML0.25 |
| J1745 |
10 mg |
800 mg |
80 |
57894003001 |
UN |
UN8 |
| J1568 |
500 mg |
40000 mg |
80 |
68982085004 |
ml |
ML400 |
| J1885 |
15 mg |
60 mg |
4 |
00409379601 |
ml |
ML2 |
| J2357 |
5 mg |
300 mg |
60 |
50242021404 |
UN |
ML2 |
| J3301 |
10 mg |
80 mg |
8 |
00003029328 |
ml |
ML2 |
| J3420 |
1000 mcg |
1000 mcg |
1 |
00517003225 |
ml |
ML1 |
| J9155 |
1 mg |
240 mg |
240 |
55566830301 |
UN |
UN3 |
| J9155 |
1 mg |
240 mg |
240 |
55566840301 |
UN |
UN2 |
| J9263 |
0.5 mg |
600 mg |
300 |
00781331570 |
If powder or oral tablet, use UN. If liquid in a vial, use ML. |
ML30 |
| J9354 |
1 mg |
160 mg |
160 |
50242008701 |
UN |
UN1 |
About National Drug Code quantities and conversions
- See the December 2018 Record for general guidelines for billing injectable drugs.
- See the June 2016 Record for tips on how to calculate and bill NDC quantities and tips on billing electronically.
- For information on a tool you can use to quickly and easily convert HCPCS or CPT units to NDC units, see the September 2017 Record.
**Billable units can be found on the Injections Minimum Fee Schedule on web-DENIS.
Blue Cross promotes coordination of care
Blue Cross Blue Shield of Michigan has a process to promote continuity and coordination of care among specialists and primary care physicians, as well as behavioral health and primary care physicians.
Collaboration among practitioners can greatly improve both member satisfaction and health outcomes.
We collect and analyze data each year to assess coordination of care and the exchange of information between specialists, behavioral health and primary care doctors following both inpatient and outpatient consultations. Many studies have identified fragmentation of care as a problem in the medical system. The information we collect is important as we work to improve continuity and coordination of care within our network.
Patient care that isn’t coordinated between providers and across settings results in:
- Confusion for members
- Increased risks to patient safety due to errors
- Unnecessary costs due to duplicate testing
Our goal for exchange of information between the specialist and the primary care doctor, as well as behavioral health provider and primary care physician, is 100 percent. This goal can be accomplished by:
- Ensuring that the specialist and behavioral health care provider have the correct primary care doctor information at the time of the visit
- Forwarding the post-visit information to the primary care doctor
- Offering behavioral health patients the opportunity to sign an authorization for release of information. If the patient declines to share this information, the provider should make a note of that on the form.
We encourage all providers to continue to take steps to enhance the information exchange across the continuum of care.
Here’s what you need to know about doing business with Blue Cross
There’s certain information our participating providers need to know about doing business with Blue Cross Blue Shield of Michigan. Each year, we provide this information to providers through The Record and web-DENIS. This article provides a summary of key information.
How to access our online provider manuals — Everything you need to know to do business with Blue Cross is included in our online provider manuals. From the homepage of web-DENIS, click on Provider Manuals to access them.
Access and availability guidelines — When a member requests an appointment, Blue Cross providers are required to comply with the following standards.
| Access to primary care |
- Regular and routine care — within 30 business days
- Urgent care — within 48 hours
- After-hours care — 24 hours, seven days a week
|
| Access to behavioral health care |
- Not life-threatening emergency — within six hours
- Urgent care — within 48 hours
- Initial visit for routine care — within 10 business days
- Follow-up routine care — within 30 business days of request
|
| Access to specialty care |
High-volume specialist including, but not limited to:
OB-GYN and high-impact specialist (oncologist) —
- Regular and routine care — within 30 business days
Urgent care — within 48 hours
|
For more detailed information, see the PPO Policies chapter in the provider manual or contact your provider consultant.
Affirmative statement about incentives — Medical decisions are based only on appropriateness of care and service and existence of coverage. See the affirmation statement in the “Participation” chapter of the provider manual. It’s located in the section titled Requirements and Guidelines.
Clinical practice guidelines — For medical and behavioral health care, Blue Cross follows Michigan Quality Improvement Consortium guidelines, which can be found on the mqic.org** website.
Comprehensive care management — To learn about Blue Cross comprehensive care management, use your online provider manual. To find the information on bcbsm.com:
- Click on the For Members tab.
- Click on Health and Wellness.
- Scroll down to Case Management or Chronic Condition Management and click on Learn More.
Criteria used for level of care utilization management decisions
For hospitals and facilities, Blue Cross uses InterQual criteria to assess medical necessity and the appropriate level of care. Criteria encompasses acute care (adult and pediatric), rehabilitation (adult and pediatric), long-term acute care, skilled nursing facility and home health care.
Blue Cross modifications of the InterQual criteria (local rules) can be accessed online by following these steps:
- Log in to web-DENIS.
- Click on BCBSM Provider Publications and Resources.
- Click on Newsletters & Resources.
- Click on Clinical Criteria & Resources.
If you have questions about InterQual, send an email to CESupport@mckesson.com. Provide your name and address and reference that the question pertains to InterQual.
Note: Criteria for Federal Employee Program® utilization management decision-making can be found at fepblue.org.
Medical policies
To review additional Blue Cross medical policies, go to bcbsm.com/providers.
- Click on Quick Links.
- Click on Preauthorization and precertification.
- Click on Medical policy, precertification and preauthorization router. Use the button to select Medical Policy, then follow online prompts.
Note: FEP policies can be found at fepblue.org.
Member rights and responsibilities
Blue Cross outlines the rights and responsibilities of our members, including how members can file a complaint or grievance. Go to the Important Information page on our website and click on Learn More under Rights and responsibilities for more information.
Pharmacy management
It’s important for you to be familiar with our drug lists and our pharmacy management programs, such as step therapy, quantity limits, dose optimization, use of generics and specialty pharmacy. You also need to know how to request prior authorization and the information needed to support your request.
Note: Generic substitution may be required for Blue Cross members. If both the generic and brand name are listed on our drug list, members are encouraged to receive the generic equivalent when available. Some members may be required to pay the difference between the brand-name and generic drug, as well as applicable copay, depending on the member’s plan. See the Pharmacy Services page on our website for more details. We recommend that you visit this page at least quarterly to access our drug lists and view updates. You can also call 1-800-437-3803 for the most up-to-date pharmaceutical information.
Translation services
Members who need language assistance should call the Customer Service number on the back of their member ID card. TTY users should call 711.
Utilization management staff availability
Department telephone numbers and hours are shown in the Preapproval Decisions/Utilization Management Decisions section of the “Appeals and problem resolution” chapter of the provider manuals.
Behavioral health care — New Directions
New Directions Behavioral Health is an independent company administering behavioral health benefits on behalf of Blue Cross. For information on the New Directions Behavioral Health Quality Improvement Program, click here.**
Contact information:
- Commercial PPO and Traditional programs: 1-800-762-2382
- Federal Employee Program: 1-800-342-5891
| Behavioral health criteria |
New Directions medical necessity criteria for behavioral health admissions are reviewed annually and updated as needed. Providers may download it at ndbh.com** or request a printed copy by contacting New Directions at 1-800-528-5763. Providers may also view or print this document by accessing via web-DENIS.
|
| Behavioral health member rights and responsibilities |
For members’ behavioral health services rights and responsibilities, click here.**
|
| Behavioral health statement about incentives |
Decisions about utilization of behavioral health services are made only on the basis of eligibility, coverage and appropriateness of care and services. New Directions doesn’t specifically reward, hire, promote or terminate practitioners or other individuals for issuing denials of coverage. Utilization decision-makers don’t receive incentives that would result in underutilization.
|
For more information
- Information about our programs and additional resources are available on the
Important Information page of our website.
- To request a printed copy of any of the information contained in this article, call Quality and Population Health at 248-455-2808.
- If you have any questions about the information in this article, contact your provider consultant.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Coding corner: Major depressive disorder
According to the National Institute of Mental Health, major depression affects about 6.7 percent of the U.S. population over age 18. Overall, between 20 to 25 percent of adults may suffer an episode of major depression at some point during their lifetime.
The Diagnostic and Statistical Manual of Mental Disorders (fifth edition) defines major depression as an individual experiencing five or more symptoms during the same two-week period, with at least one of the symptoms being either depressed mood or loss of interest or pleasure. Major depression symptoms include:
- Depressed mood most of the day, nearly every day
- Markedly diminished interest or pleasure in all, or almost all, activities most of the day, nearly every day
- Significant weight loss when not dieting, weight gain, or decrease or increase in appetite nearly every day
- A slowing down of thought and a reduction of physical movement (observable by others, not merely subjective feelings of restlessness or being slowed down)
- Fatigue or loss of energy nearly every day
- Feelings of worthlessness or excessive or inappropriate guilt nearly every day
- Diminished ability to think or concentrate, or indecisiveness, nearly every day
- Recurrent thoughts of death, recurrent suicidal ideation without a specific plan, or a suicide attempt or a specific plan for committing suicide
Documentation and coding tips
- Document major depressive disorder to the highest level of specificity, including recurrence, severity and current status:
- Episode: Single episode or recurrent
- Severity: Mild, moderate, severe, with or without psychotic symptoms
- Status: In partial remission or in full remission
- Document any underlying causes of depression, if known, such as:
- Thyroid or adrenal gland disorders
- Benign or malignant brain tumors
- AIDS
- Parkinson’s disease
- Multiple sclerosis
- Document the treatment plan
- Psychotherapy
- Antidepressant medications such as tricyclic antidepressants; monoamine oxidase inhibitors, or MAOIs; selective serotonin reuptake inhibitors, or SSRIs; or serotonin-norepinephrine reuptake inhibitors, or SNRIs.
- Electroconvulsive therapy, or ECT
More information
- A single episode of depression is commonly triggered by a life event or an underlying cause, and after a period of treatment, patients are successfully weaned off.
- Recurrent depression is typically lifelong and, although the severity may fluctuate, patients require ongoing treatment to alleviate their depression symptoms.
- With appropriate treatment, the patient’s depression symptoms may be controlled, in which case he or she is considered
in remission. The patient still, however, carries the diagnosis of major depression.
Examples of major depressive disorder codes are shown in the chart below:
| Condition |
ICD-10 code |
| Major depressive disorder, single episode, mild |
F32.0 |
| Major depressive disorder, single episode, moderate |
F32.1 |
| Major depressive disorder, single episode, severe without psychotic features |
F32.2 |
| Major depressive disorder, single episode, severe with psychotic features |
F32.3 |
| Major depressive disorder, recurrent, mild |
F33.0 |
| Major depressive disorder, recurrent, moderate |
F33.1 |
| Major depressive disorder, recurrent, severe without psychotic features |
F33.2 |
| Major depressive disorder, recurrent, severe with psychotic features |
F33.3 |
| Major depressive disorder, recurrent, in partial remission |
F33.41 |
| Major depressive disorder, recurrent, in full remission |
F33.42
|
Sources:
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations
New HEDIS CBP measure specifications eliminate need for medical record reviews
The controlling high blood pressure HEDIS® measure has been updated to assess patients ages 18 to 85 who had a diagnosis of hypertension and whose blood pressure was adequately controlled (less than 140/90) during the last reading of the year.
Previous CBP HEDIS, or Healthcare Effectiveness Data and Information Set, specifications required medical record reviews to determine if a patient’s blood pressure was under control. Now, Blood Pressure CPT Category II results codes will determine compliance.
When you add the correct CPT Category II and ICD-10 codes to your claims, you won’t need to include medical records for confirmation. This optimizes time and reduces record keeping for providers.
To learn more about claims coding to reduce medical record reviews and other measure changes, view the CBP tip sheet here.
Questions about HEDIS compliance? Go to bcbsm.com/providers for additional resources.
HEDIS® is a registered trademark of the National Committee for Quality Assurance.
Inetico managing prior authorization services for Dart PPO enrollees
On April 1, 2019, Inetico started providing prior authorizations and utilization and case management for Dart PPO enrollees’ medical plans and mental health and substance use disorders benefits. Affected members are in group number 71750.
Inetico has replaced the services of AIM Specialty Health. All service and procedure codes previously reviewed by AIM are now managed by Inetico. This includes high-tech imaging (MRI, CT, PET), in-lab sleep studies, echo cardiology and proton beam.
Inetico, a care coordination and care management provider working on behalf of Dart Container of Michigan LLC, is handling prior authorizations as well as approvals and denials for dates of service on or after April 1, 2019.
Inetico has also replaced the services of New Directions Behavioral Health for Dart PPO enrollees and is providing utilization and case management for mental health and substance use disorders benefits.
Services that require authorization by Inetico include:
Medical (except specified organ and bone marrow transplants)
- Inpatient precertification
- Preauthorization
- Air ambulance
Diagnostic testing and treatments (formerly managed by AIM)
- High-tech imaging (MRI, CT, PET)
- In-lab sleep studies
- Echo cardiology
- Proton beam
Mental health
- Inpatient
- Residential
- Partial
- Intensive outpatient
- rTMS, or repetitive transcranial magnetic stimulation
Substance use disorder
- Acute detox
- Residential
- Partial outpatient
- Intensive
For Inetico’s services, call 1-877-224-6700.
Blue Cross Blue Shield of Michigan is handling prior authorizations for the following services:
Medical benefit — drugs
Call the Pharmacy Clinical Help Desk at 1-800-437-3803.
Specified organ and bone marrow transplants
Call the Human Organ Transplant and Air Ambulance Program at 1-800-242-3504.
Dart enrollees affected by this change have received new ID cards and contact information. Blue Cross will continue to process related claims.
Always check your patients’ ID cards and web-DENIS for eligibility and prior authorization information.
Flyer helps FEP members know where to go for care
As the world of health care continues to change, options for where to get care grow. Although emergency rooms are an important part of our health care system, many people misunderstand their purpose. By accessing the right level of care, patients can save time and money.
The Federal Employee Program® offers several choices for care to its members:
- 24/7 Nurse Line
- Telehealth
- Primary care doctor
- Retail clinic
- Urgent care center
- Emergency room
Print and give the Know where to go flyer to your patients to help them determine the right choice for care

Additional Medicare Advantage post-acute care training dates set
As you read in the April Record, authorizations for Medicare Plus BlueSM PPO and BCN AdvantageSM members who are moving into skilled nursing, long-term acute care and inpatient rehabilitation facilities will be managed by naviHealth starting in June. We have a complete training schedule, detailed later in this article, to assist you with the transition.
The transition to naviHealth is effective for authorization requests submitted for admission dates on or after June 1, 2019, for both in-state and out-of-state cases. It includes members moving from acute care facilities or from another site of care.
As part of our move to naviHealth, we’re working to standardize the management of authorizations for post-acute care for Medicare Advantage members. In addition, we hope to improve the member experiences by offering a more coordinated, patient-focused approach — one that’s aimed at improving the member’s outcomes and reducing the likelihood of readmissions to an acute care setting.
What’s changing
For Medicare Plus Blue and BCN Advantage members admitted on or after June 1, 2019, you’ll submit authorization requests for skilled nursing, long-term acute care and inpatient rehabilitation to naviHealth.
You should submit these requests through the naviHealth provider portal. To access the naviHealth portal, follow these steps:
- Visit bcbsm.com/providers.
- Log in to Provider Secured Services.
- Click on Medicare Advantage Post-Acute Care Authorization on the Provider Secured Services homepage.
- Enter your NPI.
- Click on Go.
The naviHealth provider portal screen will open and you can enter your request.
If you’re unable to access the naviHealth provider portal through Provider Secured Services, call the Blue Cross Web Support Help Desk at 1-877-258-3932 for assistance.
There are other ways to submit these authorization requests to naviHealth:
- Through the naviHealth provider portal at access.navihealth.com.
Note: This option will not be available until June 1, 2019. You must first register with naviHealth for access to its portal. We’ll let you know how to do that in the training webinars.
- By phone: 1-855-851-0843
- By fax:
- Fax new authorization requests to 1-844-899-3730.
- Fax continued stay requests to 1-844-736-2980.
- Fax discharges to 1-844-729-2591.
- By email (for discharges only): mid-west_discharge_info@navihealth.com
You can also submit these requests through Allscripts. Follow your current process for submitting to Allscripts.
You can use any of the above-mentioned methods to submit authorization requests for both in-state and out-of-state members covered by Medicare Plus Blue PPO and BCN Advantage plans.
Refer to the Post-acute care services: Frequently asked questions for providers document for more detailed information.
Join us to learn more
Training sessions have been scheduled and will include information regarding the naviHealth clinical model and provider portal. Administrators, case managers, discharge planners, rehabilitation directors, nursing directors and others involved in post-acute patient care are encouraged to attend a webinar. Even if you are already familiar with naviHealth, we hope you’ll attend to learn how naviHealth will work with your Medicare Plus Blue PPO and BCN Advantage patients.
See the following table for the webinar dates and times. Click here to register.
| Acute care hospitals |
- Tuesday, May 21, 8 to 9:30 a.m.
- Wednesday, May 22, 11:30 a.m. to 1 p.m.
- Wednesday, May 29, 8 to 9:30 a.m.
- Wednesday, June 5, 8 to 9:30 a.m.
|
| Skilled nursing facilities |
- Tuesday, May 21, 11:30 a.m. to 1:30 p.m.
- Thursday, May 23, 11:30 a.m. to 1:30 p.m.
- Wednesday, May 29, 11:30 a.m. to 1:30 p.m.
- Wednesday, June 5, 11:30 a.m. to 1:30 p.m.
|
| Inpatient rehabilitation facilities and long-term acute care hospitals |
- Thursday, May 23, 8 to 9:30 a.m.
- Thursday, May 30, 11:30 a.m. to 1 p.m.
- Thursday, June 6, 11:30 a.m. to 1 p.m.
|
Note: In the April Record, we provided a separate registration link for each webinar. You can register using those links, but we encourage you to click here to find all the registration information in one location.
Skilled nursing facility in-person forums
Skilled nursing facilities are invited to attend in-person forums the week of May 13 in Traverse City, Grand Rapids, Saginaw and Southfield. For locations and to register, click here.
| Traverse City |
|
| Grand Rapids |
- Tuesday, May 14, 9-11 a.m.
- Tuesday, May 14, 1-3 p.m.
|
| Saginaw |
- Wednesday, May 15, 9-11 a.m.
- Wednesday, May 15, 1-3 p.m.
|
| Southfield |
- Thursday, May 16, 9-11 a.m.
- Friday, May 17, 9-11 a.m.
|
Reminder: Prior authorization changes to AIM authorization program for MA PPO members begin May 1, 2019
Starting May 1, 2019, certain cardiac procedures and in-lab sleep testing will require prior authorization through AIM Specialty Health for Medicare Plus BlueSM PPO members. This includes UAW Retiree Medical Benefits Trust members with Medicare Plus Blue coverage.
The PPO radiology management program added the cardiology and in-lab sleep study prior authorization program earlier this year.
The additional cardiac procedures below require prior authorization for Medicare Plus Blue members:
Percutaneous coronary intervention
- *92920
- *92924
- *92928
- *92933
- *92937
- *92943
Diagnostic coronary catheterization
Note: URMBT commercial PPO members are excluded from the prior authorization requirement for the expanded cardiology services described above.
Blue Cross Blue Shield of Michigan now requires prior authorization for in-lab sleep testing by in-state providers for Medicare Plus Blue for the following procedure codes:
- *95805
- *95807
- *95808
- *95810
- *95811
In addition, the high-tech radiology program (breast MRI) now requires Blue Cross commercial PPO and Medicare Plus Blue PPO members to get prior authorization through AIM for the procedure codes below:
How to request authorization
For more information
Refer to the February 2019 Record article or the FAQ for more information.
Medical drug prior authorization program expanding
Starting July 1, 2019, Khapzory™ and Fusilev® will be added to the Medical Drug Prior Authorization Program for Blue Cross Blue Shield of Michigan PPO commercial members.
- Khapzory (levoleucovorin sodium, HCPCS code J3490)
- Fusilev (levoleucovorin calcium, HCPCS code J0641)
This change applies to members starting therapy on or after July 1. There is nothing needed for members currently on Fusilev or Khapzory. These drugs are already included in the prior authorization program for Blue Care Network HMOSM commercial members.
The authorization requirement only applies to groups currently participating in the commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To find a list of groups that don’t take part in the program, follow these steps:
- Log in to Provider Secured Services.
- Click on BCBSM Provider Publications and Resources.
- Click on Newsletters & Resources.
- Click on Forms.
- Click on Physician administered medications.
- Click on BCBSM Medical Drug Prior Authorization Program list of groups that have opted out.
These changes don’t apply to BCN AdvantageSM, Blue Cross Medicare Plus BlueSM PPO or Federal Employee Program® members.
A prior authorization approval isn’t a guarantee of payment. Providers need to verify eligibility and benefits for members. Members are responsible for the full cost of medications not covered under their medical benefit coverage.
For a list of requirements for drugs covered under the medical benefit and for directions on how to submit a prior authorization request, see the following resources:
The new prior authorization requirement for Khapzory and Fusilev will be reflected in the medical drug list before the July 1 effective date.
Inetico managing prior authorization services for Dart PPO enrollees
On April 1, 2019, Inetico started providing prior authorizations and utilization and case management for Dart PPO enrollees’ medical plans and mental health and substance use disorders benefits. Affected members are in group number 71750.
Inetico has replaced the services of AIM Specialty Health. All service and procedure codes previously reviewed by AIM are now managed by Inetico. This includes high-tech imaging (MRI, CT, PET), in-lab sleep studies, echo cardiology and proton beam.
Inetico, a care coordination and care management provider working on behalf of Dart Container of Michigan LLC, is handling prior authorizations as well as approvals and denials for dates of service on or after April 1, 2019.
Inetico has also replaced the services of New Directions Behavioral Health for Dart PPO enrollees and is providing utilization and case management for mental health and substance use disorders benefits.
Services that require authorization by Inetico include:
Medical (except specified organ and bone marrow transplants)
- Inpatient precertification
- Preauthorization
- Air ambulance
Diagnostic testing and treatments (formerly managed by AIM)
- High-tech imaging (MRI, CT, PET)
- In-lab sleep studies
- Echo cardiology
- Proton beam
Mental health
- Inpatient
- Residential
- Partial
- Intensive outpatient
- rTMS, or repetitive transcranial magnetic stimulation
Substance use disorder
- Acute detox
- Residential
- Partial outpatient
- Intensive
For Inetico’s services, call 1-877-224-6700.
Blue Cross Blue Shield of Michigan is handling prior authorizations for the following services:
Medical benefit — drugs
Call the Pharmacy Clinical Help Desk at 1-800-437-3803.
Specified organ and bone marrow transplants
Call the Human Organ Transplant and Air Ambulance Program at 1-800-242-3504.
Dart enrollees affected by this change have received new ID cards and contact information. Blue Cross will continue to process related claims.
Always check your patients’ ID cards and web-DENIS for eligibility and prior authorization information.
|


