|
August 2023

Medicare Advantage members in crisis to get new options for behavioral health care treatment
Starting Jan. 1, 2024, our Medicare Advantage members (Medicare Plus Blue℠ and BCN Advantage℠) will have some new options for receiving help if they’re having a behavioral health crisis, as part of our crisis services program.
“These options can be used in place of going to an emergency room in an effort to hasten access to behavioral health-focused care,” said Dr. William Beecroft, medical director of behavioral health for Blue Cross Blue Shield of Michigan.
Blue Cross and Blue Care Network commercial plans began offering this program in October 2021.
Care options include:
- Psychiatric urgent care
- Mobile crisis services
- On-site crisis stabilization services
- Residential crisis treatment
Several facilities in Michigan currently offer these services as part of this program, with additional facilities expected to join the program in the future.
See our Help in times of crisis flyer for details on locations, phone numbers, service areas and care options available at these locations.
In a crisis, members or other individuals — including family members, friends, law enforcement personnel or emergency department staff — can call the number of a crisis location in their service area for guidance.
A mobile unit may be deployed to offer assessment and treatment. Walk-ins are also accepted at some locations.
“The goal of such services is to make sure our members get treated at the right place at the right time,” Dr. Beecroft said.
About our mobile crisis services
Mobile crisis services include:
- Professional mental health teams in the community who can evaluate the members wherever they are located — even in their homes, school, work or doctor’s office
- Face-to-face evaluations, telemedicine or phone evaluations to develop a treatment plan, initiate treatment and, if needed, referral to an appropriate placement for the member
The mobile crisis team may stay involved for two to four weeks after the initial encounter to ensure members are connected to the right level of care for mental health or substance use disorder treatment, and to provide treatment as necessary.
About our on-site crisis stabilization services
On-site crisis stabilization services include:
- Behavioral health evaluation to initiate appropriate treatment (similar to medical observation services)
- Physical site‑based services that are necessary to support the mobile crisis team
- Includes intake assessment, psychiatric evaluation, crisis intervention and initiation of treatment, such as psychotherapy, medication administration, therapeutic injection, observation and peer support
- Initiating coordinated linkages and “warm handoffs” to the appropriate level of care and community resources
Facilities used for physical site-based services are open 24/7. Members will have access to services from a multidisciplinary staff, including physicians, registered nurses, licensed master social workers, psychologists, clinical supervisors and additional support staff.
As part of the evaluation and treatment process at these facilities, some members may still need psychiatric hospitalization.
We’ll keep you informed as additional locations join this program.
Here’s some key information for Michigan providers who treat Medicare Plus Blue members
We’ve compiled some important information that we want to make sure health care providers who treat Medicare Plus Blue℠ members know about, including:
- Determining whether prior authorization is required for a Medicare Plus Blue member
- Submitting preservice and post-service appeals
- Submitting claims
- Changes resulting from the end of the COVID-19 public health emergency
Prior authorization
To determine whether prior authorization is required for a service for a Medicare Plus Blue member, see the document Determining prior authorization requirements for members, which you can find at ereferrals.bcbsm.com. The sections for Michigan providers include step-by-step instructions.
When submitting prior authorization requests, always include complete clinical documentation to support medical necessity.
Select elective medical and surgical procedures require prior authorization for members who reside in Michigan and use contracted Medicare Plus Blue providers. See the “Prior authorization of other medical/surgical services” section of the Medicare Plus Blue PPO Provider Manual for information about authorization criteria and medical policies.
Appeals
All Michigan providers should submit preservice and post-service appeals directly to Blue Cross Blue Shield of Michigan. Appeals should include the member’s most recent medical records. Noncontracted providers must submit a waiver of liability.
Claim submissions
Blue Cross follows the Centers for Medicare & Medicaid Services guidelines published in the Medicare Claims Processing Manual** and the Medicare National Coverage Determinations / Local Coverage Determinations.**
To reduce the chance of a claim denial:
- Refer to CMS guidelines to confirm services that require medical records and other criteria.
- Ensure that all appropriate diagnosis codes, procedure codes and modifiers (if applicable) are included on the initial claim.
- Reference CMS coding guidelines to prevent unbundling and other coding errors. Bill in the same manner as you would bill Medicare.
- Before submitting a duplicate claim, allow 30 to 45 days for the initial claim to be processed and a determination to be made.
Helpful resources
Review the following for more information on the topics addressed above:
Changes resulting from end of COVID-19 public health emergency
Many of the flexibilities and waivers that were put in place during the COVID-19 public health emergency, or PHE, ended when the PHE ended on May 11, 2023. The only changes that remain are those the U.S. government extended or made permanent — telehealth flexibilities, for example. For services provided to Blue Cross members on and after May 12, 2023, normal plan rules apply.
The following utilization management requirements resumed on July 1, 2023:
- Clinical review is required for acute medical inpatient admissions related to COVID-19, flu, pneumonia or respiratory syncytial virus.
- Standard time frames for submitting appeals of prior authorization determinations apply. Refer to the denial letter to determine the time frame.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Ruoff’s family practice: Team approach leads to high-quality care

Dr. Gary S. Ruoff, pictured with Teri Brady, family nurse practitioner, said of his practice in Traverse City: “We have a high-functioning team that operates much like a family.”
This is the fifth article in a series highlighting some top performers in the Patient-Centered Medical Home Designation Program.
Gary S. Ruoff, D.O., a family practitioner in Traverse City, firmly believes that a team approach is the best way to achieve high-quality care.
“We have a high-functioning team that operates much like a family,” he said. “As a result, we experienced very little turnover during the pandemic, which is a testament to our shared commitment to providing the best possible care to patients.”
Dr. Ruoff’s team-based approach is rooted in the Patient-Centered Medical Home model of care. The practice has been part of Blue Cross Blue Shield of Michigan’s Patient-Centered Medical Home designation program for more than 10 years.
“The PCMH Interpretive Guidelines provide a great outline for delivering high-quality care and holding the practice accountable to ensure they have a standardized and documented process to catch any patients who might otherwise fall through the cracks,” he said.
To further help patients, the practice has added care management to the array of services they provide, which has been helpful in engaging patients who need additional support and closing gaps in care. “The use of care management, registries and other PCMH tools has helped us to not only manage individual patients, but better manage the entire patient population,” he said.
Advice for other practices
The first step in implementing PCMH capabilities, Dr. Ruoff said, is to assess which capabilities are most relevant to the practice, with an end goal of improving patient outcomes, enhancing the patient experience and reducing costs. “It’s crucial to create processes and procedures with this framework in mind,” he said.
He added that building a strong team of committed staff members who work well together is crucial for success. And he recommends that practices engage with their physician organization early on to learn from others who have gone through the same journey.
Other articles in this series
Check out the following four articles, which appeared in previous issues of The Record:
If you’d like to learn more about becoming a PCMH-designated practice, talk with your physician organization or send an email to valuepartnerships@bcbsm.com.
Questionnaire updates in e-referral system
In June, we updated questionnaires in the e-referral system. We also updated the corresponding preview questionnaires on the ereferrals.bcbsm.com website.
As a reminder, we use our authorization criteria, our medical policies and your answers to the questionnaires in the e-referral system when making utilization management determinations on your authorization requests.
Updated questionnaires
We updated the following questionnaires on the date specified below:
Questionnaire |
Opens for |
Updates |
Release date |
Blepharoplasty |
- Medicare Plus Blue℠
- BCN commercial
- BCN Advantage℠
|
- Updated a question
- Added two questions
|
June 25, 2023 |
Left atrial appendage closure |
|
Added a question |
June 11, 2023 |
Left atrial appendage closure |
- Medicare Plus Blue
- BCN Advantage
|
- Updated a few questions
- Added a question
|
June 11, 2023 |
Septoplasty |
- Medicare Plus Blue
- BCN commercial
- BCN Advantage
|
|
June 25, 2023 |
Preview questionnaires
Preview questionnaires show the questions in the e-referral system so you can prepare your answers ahead of time. To access them, go to ereferrals.bcbsm.com and:
- For BCN: Click on BCN and then click on Authorization Requirements & Criteria. Scroll down and look under the Authorization criteria and preview questionnaires heading.
- For Medicare Plus Blue: Click on Blue Cross and then click on Authorization Requirements & Criteria. Scroll down and look under the Authorization criteria and preview questionnaires – Medicare Plus Blue heading.
Authorization criteria and medical policies
The Authorization Requirements & Criteria pages explain how to access the pertinent authorization criteria and medical policies.
Changes for in-lab sleep studies start Oct. 9
Prior authorization requirements for in-lab sleep studies will change for dates of service on or after Oct. 9, 2023.
For dates of service on or after Oct. 9
- For Medicare Plus Blue℠ members, prior authorization won’t be required for in‑lab sleep studies. However, if the provider is out of network for the member’s plan, then prior authorization will be required.
- For BCN Advantage℠ members, plan notification will be required to facilitate claims payment. Prior authorization won’t be required.
For dates of service before Oct. 9
Continue to submit prior authorization requests as you do now. Specifically:
- For Medicare Plus Blue members, submit prior authorization requests to Carelon Medical Benefits Management through the Carelon ProviderPortal.
Additional options for submitting requests are outlined on the Blue Cross Carelon-Managed Procedures webpage at ereferrals.bcbsm.com.
- For BCN Advantage members, submit prior authorization requests to BCN Utilization Management through the e-referral system.
- You must complete the sleep study questionnaire in the e-referral system.
Additional information
Before Oct. 9, we’ll update several documents to reflect these changes, including:
Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage prior authorizations for select services.
It’s important to monitor the metabolic effects of psychiatric medications
A column from Dr. Beecroft on this topic appeared in the May-June issue of Hospital and Physician Update. We’re reprinting it here in case you missed it.
Antipsychotic medications, along with antidepressants and mood stabilizers, have significant weight gain associated with them.
The newer medications are better than the first-generation drugs but still have this side effect. Second-generation antipsychotic medications, such as Zyprexa® and Risperdal, have the most weight gain associated with them. Seroquel, Latuda®, Abilify® and Invega® tend to cause a medium amount of weight gain, while Geodon has the least effect. In fact, some people actually lose weight while on Geodon.
Antidepressants
Antidepressants in the SSRI** class and SNRI** class also can contribute to weight gain. Wellbutrin, an atypical agent, has the least amount of weight gain associated with it; some people lose weight on it. Depakote and lithium have long been associated with weight gain. This side effect contributes to glucose intolerance and may lead to diabetes if unmonitored.
There is some evidence that metformin prevents the weight gain. Likewise, more recent information suggests semaglutide is an effective agent to assist in the prevention of metabolic syndrome and, ultimately, diabetes. Before prescribing any of these medications, doctors should discuss possible side effects with the patient and the role that diet and exercise can play in treating prediabetes.
Monitoring patients who are on antipsychotics or antidepressants
Monitoring for metabolic syndrome as outlined below is the standard of care when patients start on these medications. The American Diabetes Association suggests monitoring the following:
- Personal history (at baseline and annually)
- Weight (at baseline, 4 weeks, 8 weeks, 12 weeks, quarterly and annually)
- Waist circumference (at baseline, 12 weeks and annually)
- Blood pressure (at baseline, 12 weeks and annually)
- Fasting plasma glucose/A1c (at baseline, 12 weeks and annually)
- Fasting lipid profile (LDL, HDL, total cholesterol; at baseline, 12 weeks and annually)
If significant issues develop while the patient is on any of these medications, changing medications may be the best solution. Or if the medication (or medication combination) the patient is on is the only one that works, then treating the resulting metabolic issues aggressively may help enhance the patient’s quality of life and decrease adverse events in the future.
We encourage you to monitor the key areas outlined above, making them part of your follow-up routine with patients who are on these medications. It’s also important to help patients understand the importance of these measures and the role they play in keeping them well.
**SSRI stands for selective serotonin reuptake inhibitors while SNRI stands for serotonin and norepinephrine reuptake inhibitors.
This content is for informational purposes only and is not intended to be medical advice. This information does not substitute for professional medical advice or a consultation with a healthcare professional.
Requirements and codes changed for some medical benefit drugs
What you need to know
We’ve added requirements for some medical drugs, and this article provides an overview. Health care providers also can use our comprehensive drug lists to find the updated requirements.
Blue Cross Blue Shield of Michigan and Blue Care Network encourage proper utilization of high-cost medications that are covered under the medical benefit. As part of this effort, we maintain comprehensive lists of requirements for our members.
In April, May and June 2023, we added requirements for some medical benefit drugs. In addition, some drugs were assigned new HCPCS codes.
Changes in requirements
For Blue Cross commercial and BCN commercial members: We added prior authorization requirements, site-of-care requirements or both as follows:
HCPCS code |
Brand name |
Generic name |
Requirement |
Prior authorization |
Site of care |
J3590** |
Elfabrio® |
pegunigalsidase alfa-iwxj |
✓ |
|
J3590** |
Omisirge® |
omidubicel-onlv |
✓ |
|
J3590** |
Qalsody™ |
tofersen |
✓ |
|
J3590** |
Vyjuvek™ |
beremagene geperpavec-svdt |
✓ |
|
For Medicare Plus Blue℠ and BCN Advantage℠ members: We added prior authorization requirements as follows:
HCPCS code |
Brand name |
Generic name |
For dates of service on or after |
J3590** |
Syfovre™ |
pegcetacoplan injection |
April 3, 2023 |
J9029 |
Adstiladrin® |
nadofaragene firadenovec-vncg |
May 1, 2023 |
J3590** |
Lamzede® |
velmanase alfa |
May 1, 2023 |
Code changes
The table below shows HCPCS code changes that were effective April 1, 2023, for the medical benefit drugs we manage.
New HCPCS code |
Brand name |
Generic name |
Q5128 |
Cimerli™ |
ranibizumab-eqrn |
Q5130 |
Fylnetra® |
pegfilgrastim-pbbk |
J1411 |
Hemgenix® |
etranacogene dezaparvovec-drlb |
J1449 |
Rolvedon™ |
eflapegrastim-xnst |
J1747 |
Spevigo® |
spesolimab-sbzo |
Q5127 |
Stimufend® |
pegfilgrastim-fpgk |
C9149 |
Tzield™ |
teplizumab-mzwv |
Q5129 |
Vegzelma® |
bevacizumab-adcd |
J0218 |
Xenpozyme™ |
olipudase alfa-rpcp |
Drug lists
For additional details, see the following drug lists:
These lists are also available on the following pages of the ereferrals.bcbsm.com website:
Additional information about these requirements
We communicated these changes previously through provider alerts that contain additional details.
You can view the provider alerts on ereferrals.bcbsm.com and on our Provider Resources site, which is accessible through our provider portal, availity.com.***
Additional information about Blue Cross commercial groups
For Blue Cross commercial groups, authorization requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. A link to this list is also available on the Blue Cross Medical Benefit Drugs page of the ereferrals.bcbsm.com website.
Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don't participate in the standard prior authorization program.
Reminder
An authorization approval isn’t a guarantee of payment. Health care providers need to verify eligibility and benefits for members.
**May be assigned a unique code in the future.
***Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
How Blue Cross and BCN are handling prior authorizations for Makena following FDA announcement
In an April 6 news release,** the U.S. Food and Drug Administration announced it has withdrawn its approval of Makena® (hydroxyprogesterone caproate), HCPCS codes J1726 and J1729. The decision also applies to generic Makena products.
This means Makena and its generic products are now unapproved and can’t lawfully be distributed in interstate commerce.
For Blue Cross Blue Shield of Michigan and Blue Care Network commercial members, here’s how we’re handling prior authorizations for Makena:
- We’re honoring prior authorization requests that have already been approved for this medical benefit drug through their end dates.
- We’re denying prior authorization requests submitted for dates of service on or after April 6, 2023.
These changes apply to:
- All Blue Cross and BCN commercial members
- All versions of Makena, including compounded and generic versions
We’ve updated the pertinent drug lists to reflect this change.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
We’re changing prior authorization requirements for some weight loss drugs
Beginning Sept. 1, 2023, Blue Cross Blue Shield of Michigan and Blue Care Network will amend prior authorization coverage criteria for the brand-name weight loss medications listed below for commercial members.
- Contrave®
- Qsymia®
- Saxenda®
- Wegovy®
- Xenical®
For certain members, weight loss drugs are excluded under the pharmacy benefit.
Prior authorization and renewal criteria changes
Starting Sept. 1, we’ll shorten the initial prior authorization approval duration to four months from 12 months for commercial Blue Cross and BCN members who initiate one of these drug therapies for the first time. The duration of subsequent prior authorization renewals following initial prior authorization approval will remain unchanged and valid for 12 months for members who meet renewal requirements.
We’ll also amend the renewal criteria for these weight loss drugs. Health care providers will be required to attest that the member is actively engaged in appropriate lifestyle modifications in conjunction with weight loss therapy for continuation of coverage after the initial prior authorization expires, and for each renewal request thereafter.
For a list of prior authorization and renewal requirements for pharmacy benefit drugs, refer to our prior authorization and step therapy document at bcbsm.com/rxinfo.
Reasons for these changes
Weight loss drug therapy is highly effective when used in conjunction with appropriate lifestyle interventions, including a balanced healthy diet and exercise. Providers should follow up with patients at regular intervals after initiating weight loss pharmacotherapy to make sure they’re continuing to engage in appropriate lifestyle modifications for optimal weight loss results.
Documentation of appropriate lifestyle modifications
Providers must attest through electronic prior authorization, or ePA, that the patient has provided them with documentation to show that they’re participating in appropriate lifestyle modifications. Here are some examples of documentation and lifestyle modifications:
- Patient documentation of lifestyle modifications may include recent food logs, exercise logs or receipts to show engagement in a formal weight loss modification program.
- Appropriate lifestyle modifications may include member participation in a formal lifestyle modification program or participation in an appropriate lifestyle modification treatment plan (healthy diet and exercise) under the supervision of their provider.
Member eligibility
Not all members have weight loss drugs covered under their pharmacy benefit. Providers should determine if members are eligible before prescribing weight loss drug therapy.
Providers can call the Provider Inquiry automated response system at 1-800-344-8525 to verify eligibility for members with Blue Cross or BCN commercial coverage.
Do you know about our ‘Practice Up’ podcast series?
We wanted to remind you about our podcast series, “Practice Up,” which gives health care providers quick, easy tips for improving the patient experience.
Podcasts include the following:
- Episode 1: A Minute to Win It
- Episode 2: What Matters Most
- Episode 3: Finding Room for Feelings
- Episode 4: Rock the Wrap-up
“These podcasts give providers concrete tools they can implement that will improve the patient experience,” said Martha Walsh, M.D., senior medical director and associate chief medical officer for Provider Engagement.
They can be accessed through our provider training site by following these steps:
- Open the registration page.
- Complete the registration, which takes less than a minute. (We recommend using the same email you use to communicate with Blue Cross Blue Shield of Michigan for provider-related needs. This will become your login ID.)
- Follow the link to log in.
- Scroll down and click on the link that says Click here to locate the podcasts.
Note: If you already have access to the site, you can go directly to Step 3 to log in.
Listening to all four of the episodes — and scoring 100% on the quiz questions — will also allow you to apply for continuing medical education credit.
If you need assistance creating your login ID or navigating the site, contact ProviderTraining@bcbsm.com.
Lunch and learn webinars for physicians and coders focus on risk adjustment, coding
As a reminder, we’re offering live, 30-minute educational webinars that provide updated information on documentation and coding for common challenging diagnoses. Webinars include an opportunity to ask questions.
Here’s our upcoming schedule and tentative topics for the webinars. Each session starts at noon Eastern time. Log in to the provider training website to register for sessions that work with your schedule.
Session date |
Topic |
Aug. 16 |
Medical record documentation and coding MEAT |
Sept. 20 |
Coding tips for COPD and asthma |
Oct. 18 |
ICD-10-CM updates and changes for 2024 |
Nov. 15 |
Coding chronic kidney disease and rheumatoid arthritis |
Dec. 13 |
CPT coding scenarios for 2024 |
If you haven’t already registered for the provider training website, follow these steps:
- Click here to register.
- Complete the registration. We recommend using the same email you use to communicate with Blue Cross Blue Shield of Michigan for other needs. This will become your login ID.
Locating a session
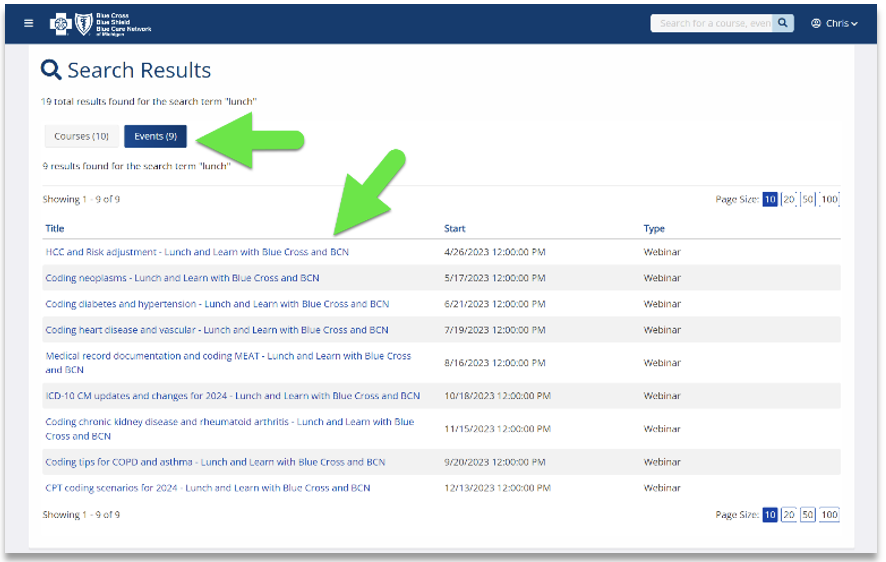
Click here if you’re already registered for the provider training website. On the provider training website, look in the Event Calendar or use the search feature using the keyword “lunch” to quickly locate all 2023 sessions.
See the screenshots below for more details.
Previous sessions
You can also listen to previously recorded sessions. Check out the following:
Date |
Topic |
April 26 |
HCC and risk adjustment coding scenarios |
May 17 |
Coding neoplasms |
June 21 |
Coding diabetes and hypertension |
July 19 |
Coding heart disease and vascular disease |
For more information
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding a session or website registration, email ProviderTraining@bcbsm.com.
New on-demand training available
Action item
Visit our provider training site to find new resources on topics that are important to you.
Provider Experience continues to offer helpful training resources for health care providers and staff. Our on-demand courses can help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network.
We recently added the following new learning opportunity to our training site:
Prior authorization basic tools — This course offers an introduction to tools and references used for submitting prior authorization requests for services, inpatient admissions and medications. Primarily designed for providers new to Blue Cross and BCN, it covers submission of prior authorization requests and a review of available tools. Search “authorization” to quickly locate the course.
Announcements and new courses, including those with CME offerings, are posted for health care providers and staff on the provider training site dashboard. Follow these steps to request access to the training site:
- Open the registration page
- Complete the registration. We recommend using the same email you use to communicate with Blue Cross for provider-related needs. This will become your login ID.
- Follow the link to log in.
If you need assistance creating your login ID or navigating the site, contact ProviderTraining@bcbsm.com.
Here are some patient resources and HEDIS tip sheets on flu vaccines and antibiotic use
This is part of an ongoing series of articles focusing on the tools and resources available to help FEP members manage their health.
To help patients understand the importance of getting the flu vaccine and why antibiotics usually don’t help for acute bronchitis, this article offers some resources for health care providers and patients.
To encourage patients to get a flu shot every year and educate them on why not taking antibiotics for acute bronchitis may be a good idea, share the following two flyers with patients:
For information on the 2023 HEDIS® measures about these topics, Blue Cross Blue Shield of Michigan developed these tip sheets for providers:
For benefit information, providers and FEP members can call Customer Service at 1-800-482-3600 or go to fepblue.org.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
HEDIS®, which stands for Healthcare Effectiveness Data and Information Set, is a registered trademark of the National Committee for Quality Assurance, or NCQA.

Submit prior authorization requests for non-emergency air ambulance flights through the Alacura PreAuth Portal
Prior to each flight, Michigan and non-Michigan health care providers must submit a prior authorization request for non-emergency air ambulance services. This requirement applies to Blue Cross Blue Shield of Michigan commercial and Blue Care Network commercial members.
Michigan’s prior authorization law requires providers to submit these requests electronically to Alacura Medical Transport Management.
Submit requests using the web form on the Alacura PreAuth Portal. To learn how to access the web form and for detailed information about completing the form, see the document titled Non-emergency air ambulance prior authorization program: Overview for Michigan and non-Michigan providers.
If you can’t access the Alacura PreAuth Portal, call Alacura or fax the Air ambulance flight information (non-emergency) form to Alacura. You can find information about these alternate submission methods in the document linked above.
You can access the overview document and the form discussed above from the Blue Cross Authorization Requirements & Criteria page or the BCN Authorization Requirements & Criteria page on the ereferrals.bcbsm.com website.
Alacura Medical Transport Management is an independent company that manages the authorization of non-emergency flights for Blue Cross Blue Shield of Michigan and Blue Care Network members who have commercial plans.
Medicare Advantage members in crisis to get new options for behavioral health care treatment
Starting Jan. 1, 2024, our Medicare Advantage members (Medicare Plus Blue℠ and BCN Advantage℠) will have some new options for receiving help if they’re having a behavioral health crisis, as part of our crisis services program.
“These options can be used in place of going to an emergency room in an effort to hasten access to behavioral health-focused care,” said Dr. William Beecroft, medical director of behavioral health for Blue Cross Blue Shield of Michigan.
Blue Cross and Blue Care Network commercial plans began offering this program in October 2021.
Care options include:
- Psychiatric urgent care
- Mobile crisis services
- On-site crisis stabilization services
- Residential crisis treatment
Several facilities in Michigan currently offer these services as part of this program, with additional facilities expected to join the program in the future.
See our Help in times of crisis flyer for details on locations, phone numbers, service areas and care options available at these locations.
In a crisis, members or other individuals — including family members, friends, law enforcement personnel or emergency department staff — can call the number of a crisis location in their service area for guidance.
A mobile unit may be deployed to offer assessment and treatment. Walk-ins are also accepted at some locations.
“The goal of such services is to make sure our members get treated at the right place at the right time,” Dr. Beecroft said.
About our mobile crisis services
Mobile crisis services include:
- Professional mental health teams in the community who can evaluate the members wherever they are located — even in their homes, school, work or doctor’s office
- Face-to-face evaluations, telemedicine or phone evaluations to develop a treatment plan, initiate treatment and, if needed, referral to an appropriate placement for the member
The mobile crisis team may stay involved for two to four weeks after the initial encounter to ensure members are connected to the right level of care for mental health or substance use disorder treatment, and to provide treatment as necessary.
About our on-site crisis stabilization services
On-site crisis stabilization services include:
- Behavioral health evaluation to initiate appropriate treatment (similar to medical observation services)
- Physical site‑based services that are necessary to support the mobile crisis team
- Includes intake assessment, psychiatric evaluation, crisis intervention and initiation of treatment, such as psychotherapy, medication administration, therapeutic injection, observation and peer support
- Initiating coordinated linkages and “warm handoffs” to the appropriate level of care and community resources
Facilities used for physical site-based services are open 24/7. Members will have access to services from a multidisciplinary staff, including physicians, registered nurses, licensed master social workers, psychologists, clinical supervisors and additional support staff.
As part of the evaluation and treatment process at these facilities, some members may still need psychiatric hospitalization.
We’ll keep you informed as additional locations join this program.
Changes for in-lab sleep studies start Oct. 9
Prior authorization requirements for in-lab sleep studies will change for dates of service on or after Oct. 9, 2023.
For dates of service on or after Oct. 9
- For Medicare Plus Blue℠ members, prior authorization won’t be required for in‑lab sleep studies. However, if the provider is out of network for the member’s plan, then prior authorization will be required.
- For BCN Advantage℠ members, plan notification will be required to facilitate claims payment. Prior authorization won’t be required.
For dates of service before Oct. 9
Continue to submit prior authorization requests as you do now. Specifically:
- For Medicare Plus Blue members, submit prior authorization requests to Carelon Medical Benefits Management through the Carelon ProviderPortal.
Additional options for submitting requests are outlined on the Blue Cross Carelon-Managed Procedures webpage at ereferrals.bcbsm.com.
- For BCN Advantage members, submit prior authorization requests to BCN Utilization Management through the e-referral system.
- You must complete the sleep study questionnaire in the e-referral system.
Additional information
Before Oct. 9, we’ll update several documents to reflect these changes, including:
Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage prior authorizations for select services.
It’s important to monitor the metabolic effects of psychiatric medications
A column from Dr. Beecroft on this topic appeared in the May-June issue of Hospital and Physician Update. We’re reprinting it here in case you missed it.
Antipsychotic medications, along with antidepressants and mood stabilizers, have significant weight gain associated with them.
The newer medications are better than the first-generation drugs but still have this side effect. Second-generation antipsychotic medications, such as Zyprexa® and Risperdal, have the most weight gain associated with them. Seroquel, Latuda®, Abilify® and Invega® tend to cause a medium amount of weight gain, while Geodon has the least effect. In fact, some people actually lose weight while on Geodon.
Antidepressants
Antidepressants in the SSRI** class and SNRI** class also can contribute to weight gain. Wellbutrin, an atypical agent, has the least amount of weight gain associated with it; some people lose weight on it. Depakote and lithium have long been associated with weight gain. This side effect contributes to glucose intolerance and may lead to diabetes if unmonitored.
There is some evidence that metformin prevents the weight gain. Likewise, more recent information suggests semaglutide is an effective agent to assist in the prevention of metabolic syndrome and, ultimately, diabetes. Before prescribing any of these medications, doctors should discuss possible side effects with the patient and the role that diet and exercise can play in treating prediabetes.
Monitoring patients who are on antipsychotics or antidepressants
Monitoring for metabolic syndrome as outlined below is the standard of care when patients start on these medications. The American Diabetes Association suggests monitoring the following:
- Personal history (at baseline and annually)
- Weight (at baseline, 4 weeks, 8 weeks, 12 weeks, quarterly and annually)
- Waist circumference (at baseline, 12 weeks and annually)
- Blood pressure (at baseline, 12 weeks and annually)
- Fasting plasma glucose/A1c (at baseline, 12 weeks and annually)
- Fasting lipid profile (LDL, HDL, total cholesterol; at baseline, 12 weeks and annually)
If significant issues develop while the patient is on any of these medications, changing medications may be the best solution. Or if the medication (or medication combination) the patient is on is the only one that works, then treating the resulting metabolic issues aggressively may help enhance the patient’s quality of life and decrease adverse events in the future.
We encourage you to monitor the key areas outlined above, making them part of your follow-up routine with patients who are on these medications. It’s also important to help patients understand the importance of these measures and the role they play in keeping them well.
**SSRI stands for selective serotonin reuptake inhibitors while SNRI stands for serotonin and norepinephrine reuptake inhibitors.
This content is for informational purposes only and is not intended to be medical advice. This information does not substitute for professional medical advice or a consultation with a healthcare professional.
Requirements and codes changed for some medical benefit drugs
What you need to know
We’ve added requirements for some medical drugs, and this article provides an overview. Health care providers also can use our comprehensive drug lists to find the updated requirements.
Blue Cross Blue Shield of Michigan and Blue Care Network encourage proper utilization of high-cost medications that are covered under the medical benefit. As part of this effort, we maintain comprehensive lists of requirements for our members.
In April, May and June 2023, we added requirements for some medical benefit drugs. In addition, some drugs were assigned new HCPCS codes.
Changes in requirements
For Blue Cross commercial and BCN commercial members: We added prior authorization requirements, site-of-care requirements or both as follows:
HCPCS code |
Brand name |
Generic name |
Requirement |
Prior authorization |
Site of care |
J3590** |
Elfabrio® |
pegunigalsidase alfa-iwxj |
✓ |
|
J3590** |
Omisirge® |
omidubicel-onlv |
✓ |
|
J3590** |
Qalsody™ |
tofersen |
✓ |
|
J3590** |
Vyjuvek™ |
beremagene geperpavec-svdt |
✓ |
|
For Medicare Plus Blue℠ and BCN Advantage℠ members: We added prior authorization requirements as follows:
HCPCS code |
Brand name |
Generic name |
For dates of service on or after |
J3590** |
Syfovre™ |
pegcetacoplan injection |
April 3, 2023 |
J9029 |
Adstiladrin® |
nadofaragene firadenovec-vncg |
May 1, 2023 |
J3590** |
Lamzede® |
velmanase alfa |
May 1, 2023 |
Code changes
The table below shows HCPCS code changes that were effective April 1, 2023, for the medical benefit drugs we manage.
New HCPCS code |
Brand name |
Generic name |
Q5128 |
Cimerli™ |
ranibizumab-eqrn |
Q5130 |
Fylnetra® |
pegfilgrastim-pbbk |
J1411 |
Hemgenix® |
etranacogene dezaparvovec-drlb |
J1449 |
Rolvedon™ |
eflapegrastim-xnst |
J1747 |
Spevigo® |
spesolimab-sbzo |
Q5127 |
Stimufend® |
pegfilgrastim-fpgk |
C9149 |
Tzield™ |
teplizumab-mzwv |
Q5129 |
Vegzelma® |
bevacizumab-adcd |
J0218 |
Xenpozyme™ |
olipudase alfa-rpcp |
Drug lists
For additional details, see the following drug lists:
These lists are also available on the following pages of the ereferrals.bcbsm.com website:
Additional information about these requirements
We communicated these changes previously through provider alerts that contain additional details.
You can view the provider alerts on ereferrals.bcbsm.com and on our Provider Resources site, which is accessible through our provider portal, availity.com.***
Additional information about Blue Cross commercial groups
For Blue Cross commercial groups, authorization requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. A link to this list is also available on the Blue Cross Medical Benefit Drugs page of the ereferrals.bcbsm.com website.
Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don't participate in the standard prior authorization program.
Reminder
An authorization approval isn’t a guarantee of payment. Health care providers need to verify eligibility and benefits for members.
**May be assigned a unique code in the future.
***Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
How Blue Cross and BCN are handling prior authorizations for Makena following FDA announcement
In an April 6 news release,** the U.S. Food and Drug Administration announced it has withdrawn its approval of Makena® (hydroxyprogesterone caproate), HCPCS codes J1726 and J1729. The decision also applies to generic Makena products.
This means Makena and its generic products are now unapproved and can’t lawfully be distributed in interstate commerce.
For Blue Cross Blue Shield of Michigan and Blue Care Network commercial members, here’s how we’re handling prior authorizations for Makena:
- We’re honoring prior authorization requests that have already been approved for this medical benefit drug through their end dates.
- We’re denying prior authorization requests submitted for dates of service on or after April 6, 2023.
These changes apply to:
- All Blue Cross and BCN commercial members
- All versions of Makena, including compounded and generic versions
We’ve updated the pertinent drug lists to reflect this change.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
We’re changing prior authorization requirements for some weight loss drugs
Beginning Sept. 1, 2023, Blue Cross Blue Shield of Michigan and Blue Care Network will amend prior authorization coverage criteria for the brand-name weight loss medications listed below for commercial members.
- Contrave®
- Qsymia®
- Saxenda®
- Wegovy®
- Xenical®
For certain members, weight loss drugs are excluded under the pharmacy benefit.
Prior authorization and renewal criteria changes
Starting Sept. 1, we’ll shorten the initial prior authorization approval duration to four months from 12 months for commercial Blue Cross and BCN members who initiate one of these drug therapies for the first time. The duration of subsequent prior authorization renewals following initial prior authorization approval will remain unchanged and valid for 12 months for members who meet renewal requirements.
We’ll also amend the renewal criteria for these weight loss drugs. Health care providers will be required to attest that the member is actively engaged in appropriate lifestyle modifications in conjunction with weight loss therapy for continuation of coverage after the initial prior authorization expires, and for each renewal request thereafter.
For a list of prior authorization and renewal requirements for pharmacy benefit drugs, refer to our prior authorization and step therapy document at bcbsm.com/rxinfo.
Reasons for these changes
Weight loss drug therapy is highly effective when used in conjunction with appropriate lifestyle interventions, including a balanced healthy diet and exercise. Providers should follow up with patients at regular intervals after initiating weight loss pharmacotherapy to make sure they’re continuing to engage in appropriate lifestyle modifications for optimal weight loss results.
Documentation of appropriate lifestyle modifications
Providers must attest through electronic prior authorization, or ePA, that the patient has provided them with documentation to show that they’re participating in appropriate lifestyle modifications. Here are some examples of documentation and lifestyle modifications:
- Patient documentation of lifestyle modifications may include recent food logs, exercise logs or receipts to show engagement in a formal weight loss modification program.
- Appropriate lifestyle modifications may include member participation in a formal lifestyle modification program or participation in an appropriate lifestyle modification treatment plan (healthy diet and exercise) under the supervision of their provider.
Member eligibility
Not all members have weight loss drugs covered under their pharmacy benefit. Providers should determine if members are eligible before prescribing weight loss drug therapy.
Providers can call the Provider Inquiry automated response system at 1-800-344-8525 to verify eligibility for members with Blue Cross or BCN commercial coverage.
Lunch and learn webinars for physicians and coders focus on risk adjustment, coding
As a reminder, we’re offering live, 30-minute educational webinars that provide updated information on documentation and coding for common challenging diagnoses. Webinars include an opportunity to ask questions.
Here’s our upcoming schedule and tentative topics for the webinars. Each session starts at noon Eastern time. Log in to the provider training website to register for sessions that work with your schedule.
Session date |
Topic |
Aug. 16 |
Medical record documentation and coding MEAT |
Sept. 20 |
Coding tips for COPD and asthma |
Oct. 18 |
ICD-10-CM updates and changes for 2024 |
Nov. 15 |
Coding chronic kidney disease and rheumatoid arthritis |
Dec. 13 |
CPT coding scenarios for 2024 |
If you haven’t already registered for the provider training website, follow these steps:
- Click here to register.
- Complete the registration. We recommend using the same email you use to communicate with Blue Cross Blue Shield of Michigan for other needs. This will become your login ID.
Locating a session
Click here if you’re already registered for the provider training website. On the provider training website, look in the Event Calendar or use the search feature using the keyword “lunch” to quickly locate all 2023 sessions.
See the screenshots below for more details.


Previous sessions
You can also listen to previously recorded sessions. Check out the following:
Date |
Topic |
April 26 |
HCC and risk adjustment coding scenarios |
May 17 |
Coding neoplasms |
June 21 |
Coding diabetes and hypertension |
July 19 |
Coding heart disease and vascular disease |
For more information
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding a session or website registration, email ProviderTraining@bcbsm.com.

HCPCS 2nd-quarter update: New and deleted codes
The Centers for Medicare & Medicaid Services has added several new codes as part of its quarterly Health Care Procedure Coding System updates. The codes, effective dates and Blue Cross Blue Shield of Michigan’s coverage decisions are below.
Injections
|
Code |
Change |
Coverage comments |
Effective date |
J0137 |
Added |
Covered |
July 1, 2023 |
J0206 |
Added |
Covered |
July 1, 2023 |
J0216 |
Added |
Covered |
July 1, 2023 |
J0457 |
Added |
Covered |
July 1, 2023 |
J0665 |
Added |
Covered |
July 1, 2023 |
J0736 |
Added |
Covered |
July 1, 2023 |
J0737 |
Added |
Covered |
July 1, 2023 |
J1440 |
Added |
Covered |
July 1, 2023 |
J1576 |
Added |
Covered |
July 1, 2023 |
J1805 |
Added |
Covered |
July 1, 2023 |
J1806 |
Added |
Covered |
July 1, 2023 |
J1811 |
Added |
Covered |
July 1, 2023 |
J1812 |
Added |
Covered |
July 1, 2023 |
J1941 |
Added |
Not covered |
July 1, 2023 |
J1961 |
Added |
Covered |
July 1, 2023 |
J2249 |
Added |
Covered |
July 1, 2023 |
J2305 |
Added |
Covered |
July 1, 2023 |
J2329 |
Added |
Covered |
July 1, 2023 |
J2371 |
Added |
Covered |
July 1, 2023 |
J2372 |
Added |
Covered |
July 1, 2023 |
J2427 |
Added |
Covered |
July 1, 2023 |
J2561 |
Added |
Covered |
July 1, 2023 |
J2598 |
Added |
Covered |
July 1, 2023 |
J2599 |
Added |
Covered |
July 1, 2023 |
J2806 |
Added |
Covered |
July 1, 2023 |
J7213 |
Added |
Covered |
July 1, 2023 |
J9381 |
Added |
Covered |
July 1, 2023 |
Q5131 |
Added |
Not covered |
July 1, 2023 |
J2370 |
Deleted |
Deleted on June 30, 2023 |
June 30, 2023 |
Injection/chemotherapy
Code |
Change |
Coverage comments |
Effective date |
J1813 |
Added |
Covered |
July 1, 2023 |
J1814 |
Added |
Covered |
July 1, 2023 |
J1836 |
Added |
Covered |
July 1, 2023 |
J1920 |
Added |
Covered |
July 1, 2023 |
J1921 |
Added |
Covered |
July 1, 2023 |
J9029 |
Added |
Covered |
July 1, 2023 |
J9056 |
Added |
Covered |
July 1, 2023 |
J9058 |
Added |
Covered |
July 1, 2023 |
J9059 |
Added |
Covered |
July 1, 2023 |
J9063 |
Added |
Covered |
July 1, 2023 |
J9259 |
Added |
Covered |
July 1, 2023 |
J9322 |
Added |
Covered |
July 1, 2023 |
J9323 |
Added |
Covered |
July 1, 2023 |
J9347 |
Added |
Covered |
July 1, 2023 |
J9350 |
Added |
Covered |
July 1, 2023 |
J9380 |
Added |
Covered |
July 1, 2023 |
S0020 |
Deleted |
Deleted on June 30, 2023 |
June 30, 2023 |
S0030 |
Deleted |
Deleted on June 30, 2023 |
June 30, 2023 |
S0073 |
Deleted |
Deleted on June 30, 2023 |
June 30, 2023 |
S0077 |
Deleted |
Deleted on June 30, 2023 |
June 30, 2023 |
Outpatient prospective payment system/injections
Code |
Change |
Coverage comments |
Effective date |
C9151 |
Added |
Covered for facility only |
July 1, 2023 |
C9149 |
Deleted |
Deleted on June 30, 2023 |
June 30, 2023 |
Outpatient prospective payment system/radiology
|
Code |
Change |
Coverage comments |
Effective date |
C9150 |
Added |
Not covered |
July 1, 2023 |
Outpatient prospective payment system/surgery
Code |
Change |
Coverage comments |
Effective date |
C9784 |
Added |
Not covered |
July 1, 2023 |
C9785 |
Added |
Not covered |
July 1, 2023 |
Outpatient prospective payment system/other medical services
|
Code |
Change |
Coverage comments |
Effective date |
C9786 |
Added |
Not covered |
July 1, 2023 |
C9787 |
Added |
Not covered |
July 1, 2023 |
Outpatient prospective payment system/injections/chemotherapy
|
Code |
Change |
Coverage comments |
Effective date |
C9146 |
Deleted |
Deleted on June 30, 2023 |
June 30, 2023 |
C9147 |
Deleted |
Deleted on June 30, 2023 |
June 30, 2023 |
C9148 |
Deleted |
Deleted on June 30, 2023 |
June 30, 2023 |
Skin substitutes
| Code |
Change |
Coverage comments |
Effective date |
Q4272 |
Added |
Not covered |
July 1, 2023 |
Q4273 |
Added |
Not covered |
July 1, 2023 |
Q4274 |
Added |
Not covered |
July 1, 2023 |
Q4275 |
Added |
Not covered |
July 1, 2023 |
Q4276 |
Added |
Not covered |
July 1, 2023 |
Q4277 |
Added |
Not covered |
July 1, 2023 |
Q4278 |
Added |
Not covered |
July 1, 2023 |
Q4280 |
Added |
Not covered |
July 1, 2023 |
Q4281 |
Added |
Not covered |
July 1, 2023 |
Q4282 |
Added |
Not covered |
July 1, 2023 |
Q4283 |
Added |
Not covered |
July 1, 2023 |
Q4284 |
Added |
Not covered |
July 1, 2023 |
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
HCPCS replacement codes, effective July 1, 2023, established
C9151 replaces C9399, J3490, J3590 and J9999 when billing for Syfovre (pegcetacoplan) for facility only
The Centers for Medicare & Medicaid Services has established a permanent procedure code for the specialty medical drug Syfovre (pegcetacoplan).
All facility services through June 30, 2023, will continue to be reported with code C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with C9151.
All professional services will continue to be reported with C9399, J3490, J3590 and J9999.
Prior authorization is still required for all groups opted into the Medical Benefit Drug program.
For groups that have opted out of the Medical Benefit Drug program, this service requires manual review.
J0457 replaces S0073 when billing for Aztreonam
CMS has established a permanent procedure code for the specialty medical drug Aztreonam.
All services through June 30, 2023, will continue to be reported with code S0073. All services performed on and after July 1, 2023, must be reported with J0457.
J0665 replaces S0020 when billing for Bupivicaine
CMS has established a permanent procedure code for the specialty medical drug Bupivicaine.
All services through June 30, 2023, will continue to be reported with code S0020. All services performed on and after July 1, 2023, must be reported with J0665.
J0736 and J0737 replace S0077 when billing for clindamycin phosphate
CMS has established a permanent procedure code for the specialty medical drug clindamycin phosphate.
All services through June 30, 2023, will continue to be reported with code S0077. All services performed on and after July 1, 2023, must be reported with J0736 or J0737.
J1836 replaces S0030 when billing for Metronidazole
CMS has established a permanent procedure code for the specialty medical drug Metronidazole.
All services through June 30, 2023, will continue to be reported with code S0030. All services performed on and after July 1, 2023, must be reported with J1836.
J1440 replaces C9399, J3490, J3590 and J9999 when billing for fecal microbiota, live – jslm
CMS has established a permanent procedure code for the specialty medical drug fecal microbiota, live – jslm.
All services through June 30, 2023, will continue to be reported with code C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J1440.
Prior authorization is required through the Medical Benefit Drug program for J1440 for all groups unless they are opted out of the program.
For groups that have opted out of the prior authorization program, this code is covered for its FDA-approved indications.
J1961 replaces C9399, J3490, J3590 and J9999 when billing for lenacapavir
CMS has established a permanent procedure code for the specialty medical drug lenacapavir.
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J1961.
J2249 replaces C9399, J3490, J3590 and J9999 when billing for reminazolam
CMS has established a permanent procedure code for the specialty medical drug reminazolam.
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J2249.
J2329 replaces C9399, J3490, J3590, J9999 when billing for ublituximab-xiiy
CMS has established a permanent procedure code for the specialty medical drug ublituximab-xiiy.
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J2329.
J2371 replaces J2370 when billing for phenylephrine hydrochloride
CMS has established a permanent procedure code for the specialty medical drug phenylephrine hydrochloride.
All services through June 30, 2023, will continue to be reported with code J2370. All services performed on and after July 1, 2023, must be reported with J2371.
J2372 replaces J2370 when billing for phenylephrine hydrochloride (Biorphen)
CMS has established a permanent procedure code for the specialty medical drug phenylephrine hydrochloride (Biorphen).
All services through June 30, 2023, will continue to be reported with code J2370. All services performed on and after July 1, 2023, must be reported with J2372.
J2427 replaces C9399, J3490, J3590 and J9999 when billing for paliperidone palmitate extended release (invega hafyera or invega trinza)
CMS has established a permanent procedure code for the specialty medical drug paliperidone palmitate extended release (invega hafyera or invega trinza).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J2427.
J2561 replaces C9399, J3490, J3590 and J9999 when billing for phenobarbital sodium (Sezaby)
CMS has established a permanent procedure code for the specialty medical drug phenobarbital sodium (Sezaby).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J2561.
J7213 replaces C9399, J3490, J3590, J9999 and J7199 when billing for IXINITY
CMS has established a permanent procedure code for the specialty medical drug IXINITY.
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and J7199. All services performed on and after July 1, 2023, must be reported with J7213.
J9029 replaces C9399, J3490, J3590 and J9999 when billing Adstiladrin (nadofaragene firadenovec-vncg)
CMS has established a permanent procedure code for the specialty medical drug Adstiladrin (nadofaragene firadenovec-vncg), a gene/cellular therapy drug.
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J9029.
J9063 replaces C9399, J3490, J3590, J9999 and C9146 when billing for ELAHERE (mirvetuximab soravtansine-gynx)
CMS has established a permanent procedure code for the specialty medical drug ELAHERE (mirvetuximab soravtansine-gynx).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9146. All services performed on and after July 1, 2023, must be reported with J9063.
J9259 replaces C9399, J3490, J3590 and J9999 when billing for American Regent (paclitaxel protein-bound particles)
CMS has established a permanent procedure code for the specialty medical drug American Regent (paclitaxel protein-bound particles).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J9259.
Prior authorization through Carelon (formerly AIM) is required for all groups opted into the Carelon prior authorization program.
For groups that aren’t in the prior authorization program, this code is covered for its FDA-approved indications.
J9322 replaces C9399, J3490, J3590 and J9999 when billing for pemetrexed (Bluepoint)
CMS has established a permanent procedure code for the specialty medical drug pemetrexed (Bluepoint).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J9322.
Prior authorization through Carelon is required for all groups opted into the Carelon prior authorization program.
For groups that aren’t in the prior authorization program, this code is covered for its FDA-approved indications.
J9323 replaces C9399, J3490, J3590 and J9999 when billing for pemetrexed ditromethamine, 10 mg
CMS has established a permanent procedure code for the specialty medical drug pemetrexed ditromethamine, 10 mg.
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J9323.
Prior authorization through Carelon is required for all groups opted into the Carelon prior authorization program.
For groups that aren’t in the prior authorization program, this code is covered for its FDA-approved indications.
J9347 replaces C9399, J3490, J3590, J9999 and C9147 when billing for Imjudo (tremelimumab-actl)
CMS has established a permanent procedure code for the specialty medical drug Imjudo (tremelimumab-actl).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9147. All services performed on and after July 1, 2023, must be reported with J9347.
J9350 replaces C9399, J3490, J3590 and J9999 when billing for Lunsumio (mosunetuzumab-axgb)
CMS has established a permanent procedure code for the specialty medical drug Lunsumio (mosunetuzumab-axgb).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J9350.
J9380 replaces C9399, J3490, J3590, J9999 and C9148 when billing for Tecvayli (teclistamab-cqyv)
CMS has established a permanent procedure code for the specialty medical drug Tecvayli (teclistamab-cqyv).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9148. All services performed on and after July 1, 2023, must be reported with J9380.
J9381 replaces C9399, J3490, J3590, J9999 and C9149 when billing for Tzield (teplizumab-mzwv)
CMS has established a permanent procedure code for the specialty medical drug Tzield (teplizumab-mzwv).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9149. All services performed on and after July 1, 2023, must be reported with J9381.
Prior authorization is required through the Medical Benefit Drug program for J9381 for all groups unless they’re opted out of the program.
For groups that have opted out of the prior authorization program, this code is covered for its FDA-approved indications.
J1576 replaces C9399, J3490, J3590 and J9999 when billing for immune globulin (Panzyga)
CMS has established a permanent procedure code for the specialty medical drug immune globulin (Panzyga).
All services through June 30, 2023, will continue to be reported with code C9399, J3490, J3590 and J9999. All services performed on and after July 1, 2023, must be reported with J1576.
Prior authorization is required through the Medical Benefit Drug program for J1576 for all groups unless they are opted out of the program.
For groups that have opted out of the prior authorization program, this code is covered for its FDA-approved indications.
J1920 replaces C9399, J3490, J3590, J9999 and C9147 when billing for labetalol hydrochloride
CMS has established a permanent procedure code for the specialty medical drug labetalol hydrochloride.
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9147. All services performed on and after July 1, 2023, must be reported with J1920.
J1921 replaces C9399, J3490, J3590, J9999 and C9147 when billing for labetalol hydrochloride (Hikma)
CMS has established a permanent procedure code for the specialty medical drug labetalol hydrochloride (Hikma).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9147. All services performed on and after July 1, 2023, must be reported with J1921.
J1805 replaces C9399, J3490, J3590, J9999 and C9147 when billing for esmolol hydrochloride
CMS has established a permanent procedure code for the specialty medical drug esmolol hydrochloride.
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9147. All services performed on and after July 1, 2023, must be reported with J1805.
J1806 replaces C9399, J3490, J3590, J9999 and C9147 when billing for esmolol hydrochloride (WG Critical Care)
CMS has established a permanent procedure code for the specialty medical drug esmolol hydrochloride (WG Critical Care).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9147. All services performed on and after July 1, 2023, must be reported with J1806.
J1813 replaces C9399, J3490, J3590, J9999 and C9148 when billing for insulin (Lyumjev) for administration through DME (i.e., insulin pump)
CMS has established a permanent procedure code for the specialty medical drug insulin (Lyumjev) for administration through durable medical equipment (i.e., insulin pump).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9148. All services performed on and after July 1, 2023, must be reported with J1813.
J1814 replaces C9399, J3490, J3590, J9999 and C9148 when billing for insulin (Lyumjev)
CMS has established a permanent procedure code for the specialty medical drug insulin (Lyumjev).
All services through June 30, 2023, will continue to be reported with codes C9399, J3490, J3590, J9999 and C9148. All services performed on and after July 1, 2023, must be reported with J1814.
Carelon Medical Benefits Management (formerly AIM Specialty Health) is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services.
Billing chart: Blue Cross highlights medical, benefit policy changes
You’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart.
This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures.
We'll publish information about new Blue Cross groups or changes to group benefits under the Group Benefit Changes heading.
For more detailed descriptions of the Blue Cross' policies for these procedures, check under the Commercial Policy tab in Benefit Explainer on Availity®. To access this online information:
1. Log in to availity.com.
2 .Click on Payer Spaces on the Availity menu bar.
3. Click on the BCBSM and BCN logo.
4. Click on Benefit Explainer on the Applications tab.
5. Click on the Commercial Policy tab.
6. Click on Topic.
7. Under Topic Criteria, click on the circle for Unique Identifier and click the drop-down arrow next to Choose Identifier Type, then click on HCPCS Code.
8. Enter the procedure code.
9. Click on Finish.
10. Click on Search.
| Code* |
BCBSM changes to:
Basic Benefit and Medical Policy, Group
Variations Payment Policy, Guidelines
|
| POLICY CLARIFICATIONS |
Established
0552T
Experimental
S8948, 97039 |
Basic benefit and medical policy
Low-level laser and high-power laser therapy
The safety and effectiveness of low-level laser therapy have been established. It may be considered a useful therapeutic option in select situations.
High-power laser therapy (nonsurgical laser) is considered experimental because evidence is insufficient to determine that the technology results in an improvement in the net health outcome.
The medical policy statement and inclusionary and exclusionary criteria have been updated, effective July 1, 2023.
Inclusions:
- LLLT when used for the prevention of oral mucositis in individuals undergoing treatment associated with increased risk of oral mucositis, including chemotherapy, radiotherapy, or hematopoietic stem cell transplantation
Exclusions:
- High-power laser therapy for all indications
- LLLT as a treatment, including as a physical therapy modality, for all other indications including but not limited to:
- Carpal tunnel syndrome
- Neck pain
- Subacromial impingement
- Adhesive capsulitis
- Temporomandibular joint pain
- Low back pain
- Osteoarthritis knee pain
- Heel pain such as Achilles tendinopathy and plantar fasciitis
- Rheumatoid arthritis
- Bell’s palsy
- Fibromyalgia
- Wound healing
- Lymphedema
|
20930, 20939, 20999,** 0565T, 0566T, 0489T, 0489T
**Unlisted procedure |
Basic benefit and medical policy
Orthopedic applications of stem-cell therapy
Mesenchymal stem cell therapy is considered experimental for all orthopedic applications, including use in repair or regeneration of musculoskeletal tissue.
Allograft bone products containing viable stem cells, including, but not limited to, demineralized bone matrix, or DBM, with stem cells, are considered experimental for all orthopedic applications.
Allograft or synthetic bone graft substitutes that must be combined with autologous blood or bone marrow are considered experimental for all orthopedic applications.
These therapies haven’t been scientifically demonstrated to improve patient clinical outcomes.
The medical policy statement has been updated, effective July 1, 2023.
Inclusionary and exclusionary guidelines:
Not applicable |
22899**
**Unlisted code |
Basic benefit and medical policy
Growing rods for scoliosis
The safety and effectiveness of FDA-approved growing rods in the treatment of early-onset scoliosis have been established. It may be considered a useful therapeutic option when indicated.
Inclusionary criteria have been updated, effective July 1, 2023.
Inclusions:
Use of FDA-approved growing rods in the treatment of early-onset scoliosis may be a therapeutic option when:
- Skeletally immature patients 10 years of age or less
- Severe progressive spinal abnormalities (e.g., Cobb angle of 30 degrees or more)
- Thoracic spine height less than 22 cm
- Curve progression despite compliance with bracing or intolerance to brace use
- Associated with or at risk of Thoracic insufficiency syndrome, or TIS**
**TIS is defined as the inability of the thorax to support normal respiration or lung growth.
Exclusions:
When the above criteria aren’t met. |
43644, 43645, 43770, 43771, 43772, 43773, 43774, 43775, 43843, 43845, 43846, 43847, 43848, 43886, 43887, 43888, 43999, 44130, 96130, 96131, 96136, 96137, 96138, 96139, S2083
Experimental
43999,** 96146, 43290, 43291, 43842
**When used to indicate any of the following procedures:
- Loop gastric bypass gastroplasty, also known as mini-gastric bypass
- Stomach stapling
- SADI-S
- SIPS
- Endoscopic procedures to treat weight gain after bariatric surgery
- Natural Orifice Transluminal Endoscopic Surgery, known as NOTES™
|
Basic benefit and medical policy
Bariatric surgery
The safety and effectiveness of laparoscopic and open gastric restrictive procedures including, but not limited to, Roux-en-Y gastric bypass, sleeve gastrectomy, biliopancreatic diversion with duodenal switch and adjustable gastric band have been established. They may be considered useful therapeutic options when specified criteria are met.
Inclusionary and exclusionary criteria have been updated, effective July 1, 2023.
Inclusions:
Surgical procedures are considered established treatment options if all the following criteria are met:
- The individual has one of the following:
- A BMI of >40
- A BMI of >35 with one or more co-morbid conditions including, but not limited to:
- Degenerative joint disease (including degenerative disc disease)
- Hypertension
- Hyperlipidemia, coronary artery disease
- Presence of other atherosclerotic diseases
- Sleep apnea
- Congestive heart failure
or
- A BMI of > 30 with Type 2 diabetes
- All individuals 18 to 60 years of age with conditions above
- Individuals older than 60 years of age may be considered if it’s documented in the medical record that the individual’s physiologic age and co-morbid conditions result in a positive risk/benefit ratio.
- Criteria for bariatric surgery for individuals younger than 18 years of age are similar: 1) BMI ≥40 kg/m2 (or 140% of the 95th percentile for age and sex, whichever is lower); 2) BMI ≥35 kg/m2 (or 120% of the 95th percentile for age and sex, whichever is lower) with clinically significant comorbidities; and should include documentation that the primary care provider has addressed the risk of surgery on future growth, the patient's maturity level and the patient’s ability to understand the procedure and comply with postoperative instructions, as well as the adequacy of family support.
- The individual has undergone multidisciplinary evaluation by an established bariatric treatment program to include medical, nutritional and mental health evaluations to determine ultimate candidacy for bariatric surgery. Such an evaluation should include an assessment of the patient’s likely ability and willingness to cooperate effectively with a rigorous post-operative program. This should include documentation of past participation in a non-surgical weight-loss program. Documentation of a non-surgical weight-loss program is waived for super morbidly obese individuals who have a BMI of ≥50.
- The non-surgical program participation and multidisciplinary evaluation must have occurred within four years of the date of surgery.
- A psychological evaluation must be performed as a pre-surgical assessment by a contracted mental health professional to establish the patient’s emotional stability, ability to comprehend the risk of surgery and to give informed consent, and ability to cope with expected post-surgical lifestyle changes and limitations. Such psychological consultations may include one unit total of psychological testing for purposes of personality assessment (e.g., the MMPI-2 or adolescent version, the MMPI-A).
- In cases where a revision of the original procedure is planned because of failure due to anatomic or technical reasons (e.g., obstruction, staple dehiscence, etc.), or excessive weight loss of 20% or more below ideal body weight, the revision is determined to be medically appropriate without consideration of the initial preoperative criteria. The medical records should include documentation of:
- The date and type of the previous procedure
- The factors that precipitated the failure or the nature of the complications from the previous procedure that mandate (necessitate) the takedown
- If the indication for the revision is a weight gain or a failure of the patient to lose a desired amount of weight due to patient non-adherence, then the patient must re-qualify for the subsequent procedure and meet all the initial preoperative criteria.
Exclusions:
The following surgical procedures are considered experimental because their safety or effectiveness hasn’t been proven:
- Loop gastric bypass gastroplasty using a Billroth II type of anastomosis, also known as mini-gastric bypass
- Biliopancreatic bypass without duodenal switch
- Long-limb gastric bypass procedure (i.e., >150 cm)
- Stomach stapling (vertical banded gastroplasty)
- Endoscopic and endoluminal procedures (including but not limited to insertion of the StomaphyX™ device, use of the Overstitch device, insertion of a gastric balloon, endoscopic gastroplasty, intragastric balloons, aspiration therapy device) or use of an endoscopically placed duodenojejunal sleeve) as a primary bariatric procedure or as a revision procedure, (i.e., to treat weight gain after bariatric surgery to remedy large gastric stoma or large gastric pouches).
- Any bariatric surgery for individuals with Type 2 diabetes who have a BMI of less than 30
- Laparoscopic gastric plication
- Vagus nerve blocking (see the separate policy, “Vagus Nerve Blocking for Morbid Obesity.”)
- Single anastomosis duodenoileal bypass with sleeve gastrectomy, or SADI-S
- Stomach intestine pylorus sparing surgery, or SIPS
- Bariatric surgery for pre-adolescents
- Natural orifice transluminal endoscopic surgery, or NOTES™
- Two-stage bariatric surgery procedures (e.g., SG as the initial procedure followed by BPD at a later time)
|
76390
Experimental:
0609T, 0610T, 0611T, 0612T |
Basic benefit and medical policy
Magnetic resonance spectroscopy
The safety and effectiveness of magnetic resonance spectroscopy have been established for individuals who meet specific selection criteria.
Exclusionary criteria have been updated, effective July 1, 2023.
Inclusions:
Magnetic resonance spectroscopy, or MRS, is an appropriate clinical tool for diagnosing:
- Disorders of creatine metabolism
- Presence of mitochondrial disease
- MRS may be used to assist in distinguishing tissue necrosis from persistent or recurrent brain tumor as an alternative to invasive brain biopsy
Exclusions:
Use of MRS for any of the following:
- Breast cancer
- Discogenic pain (cervical, thoracic, or lumbar)
- Dementia
- Disease tracking of diagnosis including:
- Systemic lupus erythematosus
- Assessing carotid plaque morphology
- Identifying biomarkers of traumatic brain injury
- Predicting long-term neurodevelopmental outcome after neonatal encephalopathy
- Evaluation of neuroinhibitory and neuroexcitatory processes relating to pain
- Liver disease
- Multiple sclerosis
- Prostate cancer
- Psychiatric disorders including, but not limited to:
- Depression
- Bipolar disorder
- Schizophrenia
- Post-traumatic stress disorder
- Any condition other than those listed in the Inclusions section
|
81235, 81275, 81404, 81405, 81406,
81445, 81479, 0037U |
Basic benefit and medical policy
Somatic biomarker testing for targeted treatment of NSCLC
The medical policy statements regarding medically necessary testing of somatic variants used to determine treatment of non-small cell lung cancer have been updated, effective July 1, 2023.
EGFR testing
- The safety and effectiveness of analysis of somatic variants in exons 18 (such as G719X), 19 (such as L858R, T790M), 20 (such as S678I) or 21 (such as L861Q) within the EGFR gene have been established to predict treatment response to an FDA-approved therapy (e.g., erlotinib [Tarceva®], gefitinib [Iressa®] or afatinib [Gilotrif®]), or osimertinib (Tagrisso) in individuals with advanced lung adenocarcinoma, large cell carcinoma, advanced squamous cell NSCLC and NSCLC not otherwise specified, if the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label
- Analysis of tumor tissue for somatic variants in exon 20 (e.g., insertion variants) within the EGFR gene, may be considered established to predict treatment response to an FDA-approved therapy (e.g., mobocertinib [Exkivity] or amivantamab [Rybrevant]) in individuals with NSCLC, if the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label
- At diagnosis, analysis of plasma for somatic variants in exons 19 through 21 (e.g., exon 19 deletions, L858R, T790M) within the EGFR gene, using the cobas EGFR Variant Test v2, Guardant360 CDx test, FoundationOne Liquid CDx, OncoBEAM test or InVisionFirst-Lung test to detect circulating tumor DNA (ctDNA) may be considered established as an alternative to tissue biopsy to predict treatment response to an FDA-approved therapy in individuals with advanced lung adenocarcinoma, large cell carcinoma, advanced squamous cell NSCLC and NSCLC not otherwise specified if the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label
- At progression, analysis of plasma for the EGFR T790M resistance variant for targeted therapy with osimertinib using the cobas EGFR Variant Test v2, Guardant360 CDx test, OncoBEAM test, or InVisionFirst-Lung test to detect ctDNA, may be considered established in individuals with advanced lung adenocarcinoma, large cell carcinoma, advanced squamous cell NSCLC and NSCLC not otherwise specified, when tissue biopsy to obtain new tissue isn’t feasible (e.g., in those who don’t have enough tissue for standard molecular testing using formalin-fixed paraffin-embedded tissue, don’t have a biopsy-amenable lesion or can’t undergo biopsy), and when the individual doesn’t have any FDA-labeled contraindications to osimertinib and it’s intended to be used consistently with the FDA-approved label
- Analysis of plasma for somatic variants in exon 20 (e.g., insertion variants) within the EGFR gene using an FDA-approved companion diagnostic plasma test to detect ctDNA may be considered established as an alternative to tissue biopsy to predict treatment response to an FDA-approved therapy in individuals in NSCLC (e.g., amivantamab [Rybrevant]), if the individual doesn’t have any FDA-labeled contraindications to the requested agent and both the agent and ctDNA test are intended to be used consistently with their FDA-approved labels
- The analysis for other EGFR variants within exons 22-24, or other applications related to NSCLC, is considered experimental. The peer-reviewed medical literature hasn’t yet demonstrated the clinical utility of this testing for this indication.
ALK testing
- The safety and effectiveness of analysis of somatic rearrangement variants of the ALK gene in tissue have been established. It’s an effective diagnostic option for predicting treatment response to crizotinib (Xalkori®), ceritinib (Zykadia™, alectinib [Alecensa], brigatinib [Alunbrig]) or lorlatinib [Lorbrena] in patients with advanced lung adenocarcinoma and large cell carcinoma or for patients in whom an adenocarcinoma component can’t be excluded, if the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label
- Analysis of plasma for somatic rearrangement variants of the ALK gene using an FDA-approved companion diagnostic plasma tests to detect ctDNA is considered established as an alternative to tissue biopsy to predict treatment response to an FDA-approved ALK inhibitor therapy in individuals with NSCLC (e.g., alectinib [Alcensa]), if the individual doesn’t have any FDA-labeled contraindications to the requested agent and both the agent and ctDNA test are intended to be used consistently with their FDA-approved labels
- Analysis of somatic rearrangement variants of the ALK gene in tissue or plasma is considered experimental in all other situations.
BRAF V600E testing
- Analysis of the BRAF V600E variant is established to predict treatment response to FDA-approved BRAF or MEK inhibitor therapy (e.g., dabrafenib [Tafinlar] and trametinib [Mekinist®]), in individuals with advanced lung adenocarcinoma or in whom an adenocarcinoma component can’t be excluded, if the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label.
- Analysis of tumor tissue for the somatic BRAF V600E variant is considered experimental in all other situations.
- Analysis of plasma for the somatic BRAF V600E variant to detect ctDNA is considered experimental as an alternative to tissue biopsy to predict treatment response to BRAF or MEK inhibitor therapy in patients with NSCLC.
ROS1 testing
- Analysis of somatic rearrangement variants of the ROS1 gene is established to predict treatment response to FDA-approved ROS1 inhibitor therapy (crizotinib [Xalkori]) in individuals with advanced lung adenocarcinoma or in whom an adenocarcinoma component can’t be excluded. If the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label
- Analysis of tumor tissue for somatic rearrangement variants of the ROS1 gene is considered experimental in all other situations
- Analysis of plasma for somatic rearrangement variants of the ROS1 gene using plasma specimens to detect ctDNA is considered experimental as an alternative to tissue biopsy to predict treatment response to ROS1inhibitortherapy (e.g., crizotinib [Xalkori] or entrectinib [Rozlytrek]) in patients with NSCLC
KRAS testing
- Analysis of somatic variants of the KRAS gene is established as a technique to predict treatment nonresponse to sotorasib (Lumakras) in individuals with advanced lung adenocarcinoma or in whom an adenocarcinoma component can’t be excluded, if the individual doesn’t have an FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA approved label
- Analysis of plasma for somatic variants of the KRAS gene (e.g., G12C) using an FDA-approved companion diagnostic plasma test to detect ctDNA is considered established as an alternative to tissue biopsy to predict treatment response to sotorasib (Lumakras) in patients with advanced lung adenocarcinoma or in whom an adenocarcinoma component can’t be excluded.
- All other uses of analysis of somatic variants of the KRAS gene in tissue or plasma are considered experimental.
HER2 testing
- Analysis of tumor tissue for somatic alterations in the HER2 (ERBB2) gene is considered established to predict treatment response to an FDA-approved therapy (e.g., fam-trastuzumab deruxtecan-nxki [Enhertu]) in individuals with unresectable or metastatic NSCLC, if the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label.
- Analysis of plasma for somatic alterations in the HER2 (ERBB2) gene using an FDA-approved companion diagnostic plasma to detect ctDNA is considered established as an alternative to tissue biopsy to predict treatment response to an FDA-approved therapy (e.g., fam-trastuzumab deruxtecan-nxki [Enhertu]) in individuals with unresectable or metastatic NSCLC, if the individual doesn’t have any FDA-labeled contraindications to the requested agent and both the agent and ctDNA test are intended to be used consistently with their FDA-approved labels.
- All other uses of analysis of somatic variants of the HER2 (ERBB2) gene in tissue or plasma are considered experimental.
NTRK gene fusion testing
- Larotrectinib and entrectinib are considered established when all the following are met:
- Individual has a confirmatory diagnosis of a solid tumor that is metastatic or when surgical resection is likely to result in severe morbidity.
- The tumor has an NTRK gene fusion without a known acquired resistance variant.
- Individual has progressed following standard of care or failed standard of care for the given solid tumor.
- Must be prescribed by an oncologist or hematologist.
- Individual doesn’t have any FDA-labeled contraindications to the requested agent and is intended to be used consistently with the FDA-approved label.
- Larotrectinib and entrectinib are considered experimental in all other situations.
RET rearrangement testing
- Analysis of tumor tissue for somatic alterations in the RET gene may be considered established to predict treatment response to pralsetinib or selpercatinib in individuals with metastatic NSCLC if the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label.
- Analysis of tumor tissue for somatic alterations in the RET gene is considered experimental in all other situations.
- Analysis of plasma for somatic alterations of the RET gene using plasma specimens to detect ctDNA is considered experimental as an alternative to tissue biopsy to predict treatment response to RET inhibitor therapy (e.g., selpercatinib [Retevmo], pralsetinib [Gavreto]) in individuals with NSCLC.
MET exon 14 skipping alteration
- Analysis of tumor tissue for somatic alterations in tissue that leads to MET exon 14 skipping may be considered established to predict treatment response to capmatinib in individuals with metastatic NSCLC, if the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label.
- Analysis of plasma for somatic alteration that leads to MET exon 14 skipping using an FDA-approved companion diagnostic plasma tests to detect ctDNA is considered established as an alternative to tissue biopsy to predict treatment response to MET inhibitor therapy (e.g., capmatinib [Tabrecta]) in patients with NSCLC, if the individual doesn’t have any FDA-labeled contraindications to the requested agent and both the agent and ctDNA test are intended to be used consistently with their FDA-approved labels.
- Analysis of somatic alterations of the MET gene in tissue or plasma is considered experimental in all other situations.
PD-L1 testing
- PD-L1 testing may be considered established to predict treatment response to an FDA-approved therapy (e.g., atezolizumab,nivolumab in combination with ipilimumab or pembrolizumab [Keytruda], or cemiplimab-rwlc [libtayo]) in individuals with NSCLC if the individual doesn’t have any FDA-labeled contraindications to the requested agent and the agent is intended to be used consistently with the FDA-approved label.
- PD-L1 testing is considered experimental in all other situations.
Tumor mutational burden testing
- May be established for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden-high (TMB-H) [≥10 mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options (for example, Keytruda).
Plasma testing when tissue is insufficient
Plasma tests for oncogenic driver variants deemed medically necessary on tissue biopsy may be considered established to predict treatment response to targeted therapy for individuals meeting all the following criteria:
- Individual doesn’t have sufficient tissue for standard molecular testing using formalin-fixed paraffin-embedded tissue.
- Follow-up tissue-based analysis is planned should no driver variant be identified via plasma testing.
Guidelines:
Testing
Next-generation sequencing, or NGS, with a multiple-gene panel test may be considered established when used for diagnostic and prognostic purposes or for guidance in the selection of appropriate FDA-approved therapeutic options.
Proprietary laboratory analyses, or PLA, testing
A PLA test as an FDA-approved companion diagnostic to determine the appropriate therapeutic drug is considered established when all the following criteria are met:
- Biomarker confirmation is required by an FDA-approved or FDA-cleared test prior to initiating treatment (as described in the FDA prescribing label of the therapeutic in the section “Indications and Usage”).
- The test is an FDA-approved companion diagnostic.
- The FDA hasn’t identified a non-PLA test (e.g., an FDA companion diagnostic that is billed by a CPT code) for the same therapeutic indication.
FDA-approved companion diagnostic tests
FDA-approved companion diagnostic tests include:
- Tests that are billed with CPT codes (Note: Most laboratories are able to process these.)
- Proprietary laboratory analyses tests (processed by one specific independent laboratory). Most PLA tests have billing codes that end in “U.”
|
Established
81302, 81303, 81304, 81404, 81405, 81406
Experimental
0234U
|
Basic benefit and medical policy
Genetic testing for Rett syndrome
The safety and effectiveness of genetic testing for Rett syndrome have been established. It may be considered a useful diagnostic option for select individuals.
Inclusionary criteria have been updated, effective July 1, 2023.
Inclusions:
- When testing is performed to confirm a diagnosis of Rett syndrome in a child with developmental delay and signs and symptoms of Rett syndrome but when there is uncertainty in the clinical diagnosis (e.g., MECP2, FOXG1, or CDKL5)
- Targeted genetic testing for a known familial Rett syndrome-associated variant to determine carrier status of first-degree female relatives of an individual with Rett syndrome
Exclusions:
- All other indications for genetic testing for Rett syndrome-associated genes (e.g., MECP2, FOXG1 or CDKL5), including carrier testing (preconception or prenatal) and testing of asymptomatic family members to determine future risk of disease
|
81401, 81405, 81406
Experimental procedures:
S3852, 0346U
|
Basic benefit and medical policy
Genetic testing for Alzheimer’s
Genetic testing for a known familial variant in the presenilin genes, or PSEN, or amyloid-beta precursor protein, or APP, gene associated with autosomal dominant early-onset Alzheimer’s disease in an asymptomatic individual to determine future risk of disease is considered established only for those individuals meeting patient selection criteria and who are seeking preconception genetic counseling.
Genetic testing for variants in presenilin genes or amyloid-beta precursor protein gene associated with autosomal dominant Alzheimer’s disease in an asymptomatic individual to determine future risk of disease is established for individuals who meet patient selection criteria and who are seeking preconception genetic counseling
Genetic testing for confirming a diagnosis of Alzheimer’s disease or determining the risk assessment of developing Alzheimer’s when family planning isn’t an issue is considered experimental.
Exclusionary criteria have been updated, effective July 1, 2023.
Inclusions:
- Targeted genetic testing for known familial variant in the presenilin genes or amyloid-beta precursor protein gene associated with autosomal dominant early-onset Alzheimer’s disease is established when all the following criteria are met:
- The individual has a close relative (first- or second-degree relative) with a known familial variant associated with autosomal dominant early-onset Alzheimer’s disease.
- Results of testing will inform reproductive decision-making.
- Genetic testing for variants in presenilin genes or amyloid-beta precursor protein gene associated with autosomal dominant early-onset Alzheimer’s disease is established in an asymptomatic individual to determine future risk of disease when the following criteria are met:
- The individual has a family history of dementia consistent with autosomal dominant Alzheimer’s disease for whom the genetic status of the affected family members is unavailable.
- Results of testing will inform reproductive decision-making
Genetic counseling by appropriately trained individuals is strongly encouraged to be done in conjunction with the genetic testing for Alzheimer’s disease when the above criteria are met.
Exclusions:
Genetic testing for the risk assessment of Alzheimer’s disease in asymptomatic individuals is considered experimental in all other situations. Genetic testing includes, but isn’t limited to, testing for the apolipoprotein E ε4 allele, or APOE, or triggering receptor expressed on myeloid cells 2, or TREM2.
Genetic testing to guide the initiation or management of a U.S. Food and Drug Administration-approved amyloid-beta targeting therapy (e.g., aducanumab) is considered experimental. Genetic testing includes, but isn’t limited to, testing for the APOE epsilon 4 allele. |
Established
81500, 81503, 0003U
Experimental
0375U |
Basic benefit and medical policy
Multimarker serum testing for ovarian cancer
The safety and effectiveness of proteomics-based testing (e.g., OVA1®, Overa™ and ROMA™ tests) to identify women with adnexal masses who may benefit from referral to a gynecologic-oncology specialist have been established. These tests may be considered a useful (but not mandatory) diagnostic option in guiding referral to a gynecologic oncologist for women meeting defined criteria.
OvaWatch doesn’t provide any additional clinically relevant information in the diagnosis or treatment of ovarian cancer. The test is therefore considered experimental.
The medical policy statement and exclusionary criteria have been updated, effective July 1, 2023.
Inclusions:
The proteomics-based OVA1® test, Overa™ and the Risk of Ovarian Malignancy Algorithm (HE4 EIA + ARCHITECT CA 125 II), known as ROMA™, tests are considered established when used as an aid to further assess the likelihood that malignancy is present when the physician’s (other than gynecologic oncologist) independent clinical and radiological preoperative evaluations don’t indicate malignancy in a woman with an ovarian (adnexal) mass when all the following criteria have been met:
- The woman should be older than age 18 years.
- Ovarian adnexal mass is present.
- Surgery is planned for treatment of the mass.
- The patient hasn’t yet been referred to a gynecologic oncologist and referral to gynecologic oncologist is being considered in the event of a positive test result.
Exclusions:
Testing for Ova1®, Overa™ and ROMA™ that doesn’t meet the above criteria including, but not limited to:
- Screening for ovarian cancer
- Selecting patients for surgery for an adnexal mass
- Evaluation of patients with clinical or radiologic evidence of malignancy
- OvaWatch testing
|
92507, 92508, 92521, 92522, 92523, 92524, 92526, 92610 |
Basic benefit and medical policy
Speech and language pathology rehab
The effectiveness of speech and language pathology services (including voice therapy, swallowing and feeding therapy) has been established. It’s a useful therapeutic option for patients meeting patient selection criteria, effective July 1, 2023.
Inclusionary and exclusionary guidelines:
- Speech and language pathology
- Rehabilitative therapy
Inclusions (must meet all):
Outpatient speech and language rehabilitation is covered when:
- The speech therapy is ordered for the treatment of an organic medical condition resulting from illness, injury, surgery or congenital abnormality, or for the immediate postoperative or convalescent state of the patient’s illness.
- Based on a plan of care, the therapy sessions achieve a specific diagnosis-related goal for an individual who has a reasonable expectation of achieving measurable significant functional improvement in a reasonable and predictable period of time and the therapy sessions provide specific, effective and reasonable treatment for the individual’s diagnosis and physical condition and require the judgment, knowledge and skills of a qualified provider of SLP services due to the complexity and sophistication of the therapy and the medical condition of the individual.
- The treatment is provided or supervised by a licensed speech-language pathologist that is properly contracted with Blue Cross Blue Shield of Michigan.
- Coverage is also available for treatment of an acute exacerbation of a chronic condition that is subject to significant improvement.
Conditions for which rehabilitative speech and language pathology services may be covered include, but aren’t limited to:
- Intensive treatment of a physical disease, trauma, congenital anomalies, therapeutic surgical interventions (e.g., surgery to the neck or throat) or medical interventions (e.g., post- radiation therapy that affects voice, speech, language, fluency, swallowing or cognition).
- Treatment of apraxia or dyspraxia of speech
- Rehabilitation following stroke or transient ischemic attacks
- Rehabilitation post cochlear implantation (aural, speech, language and voice rehabilitation)
- Voice therapy for the pre-surgical or nonsurgical treatment of vocal cord nodules or polyps, vocal cord paresis/paralysis and muscle tension dysphonia
- Speech and language therapy for a documented diagnosis of autism as part of a comprehensive treatment program for autism. (Note: Michigan mandates treatment coverage for the diagnosis and treatment of autism spectrum disorders, including therapeutic care, e.g., services provided by a licensed or certified speech and language pathologist.)
- Speech and language therapy for developmental conditions, including syntactic, semantic or articulation disorders when it’s determined that the condition is severe and not likely to be “self-correcting.”
The following criteria help to identify severe developmental conditions.
Severity criteria for developmental conditions
- The child’s communicative abilities are scored within the severe range on a standardized test of communicative dysfunction.
- The child’s communicative abilities are scored within the severe range on a subtest of a standardized test of communicative dysfunction.
- The child is essentially nonverbal and has a communication deficit that is too severe for standardized testing.
- The child tests at more than one year behind norms for receptive language skills on a standardized test of communicative dysfunction.
- The child tests at more than one year behind for expressive language skills on a standardized test of communicative dysfunction.
- The child tests at more than one year behind norms for articulation proficiency on a standardized test of communicative dysfunction.
If a child’s severity status changes as a consequence of treatment while therapy is in progress, coverage will continue for the remainder of the 60 calendar days or 60 treatments, depending on the member’s benefit.
The medical chart must demonstrate specific treatment goals based on the original and ongoing assessment of the child’s speech or language disorder. Measurement of progress to goals must be documented.
Exclusions:
Check individual certificates relating to exclusions for the diagnosis of developmental disorders. If developmental disorders aren’t specifically listed as excluded conditions in the certificate or contract, then speech therapy may be considered for coverage for this diagnosis if criteria regarding severity are met.
- Longstanding chronic conditions where improvement is unlikely
- Therapy for conditions that are self-correcting with no history of a medical problem such as:
- Language therapy for very young children
- Mild developmental articulation errors that are self-correcting
- Developmental disorders not felt to be severe
- The expectation doesn’t exist that the speech therapy will result in a practical improvement in the level of functioning within a reasonable and predictable time period
- Summer speech therapy programs normally provided by the school system during the regular school year
- Self-correcting conditions, including hoarseness when no medical problem is identified
- Services that don’t require the skills of a qualified provider of SLP services including, but not limited to, the following:
- Treatments that maintain function using routine, repetitious or reinforced procedures that are neither diagnostic nor therapeutic (for example, practicing word drills for developmental articulation errors)
- Procedures that may be carried out effectively by the individual, family or caregivers
- Treatments that aren’t supported in peer-reviewed literature
- Voice therapy associated with gender-affirming services
Habilitative therapy
Inclusions (must meet all of the following):
- The therapy is intended to maintain, develop or improve speech, voice, language or swallowing impairment skills that haven’t (but normally would have) developed or that are at risk of being lost as a result of illness,** injury, loss of a body part or congenital abnormality. An individual would either not be expected to develop the function or would be expected to permanently lose the function without the habilitative service (not merely fluctuate).
- The therapy documentation objectively verifies that, at a minimum, functional status is maintained.
- The services require the judgment, knowledge and skills of a qualified SLP due to the complexity and sophistication of the therapy and the medical condition of the individual.
**Note: Illness includes a wide range of conditions. For purposes of clarity, illness includes, but isn’t limited to, autism spectrum disorder.
Exclusions:
- The therapy is aimed at developing, improving or maintaining functions, which would normally develop with no intervention.
- The therapy is aimed at a function that would be permanently lost as a result of illness, injury, loss of a body part or congenital abnormality whether or not therapy was provided.
- The therapy is considered primarily educational.
- The expectation doesn’t exist that the speech therapy will result in a practical improvement in the level of functioning within a reasonable and predictable period of time or an assessment of progress to goals doesn’t demonstrate “maintenance” of status or capabilities.
- Services that don’t require the skills of a qualified provider of SLP include, but aren’t limited to, the following:
- Treatments that maintain function using routine, repetitious or reinforced procedures that are neither diagnostic nor therapeutic (e.g., practicing word drills for developmental articulation errors)
- Procedures that may be carried out effectively by the individual, family or caregivers
- Routine reevaluations not meeting the above criteria
- Treatments that aren’t supported in peer-reviewed literature
- Duplicate habilitative therapy
- Longstanding chronic conditions
Swallowing therapy
Inclusions:
- Infants who have a history of being fed through a feeding tube and have never learned to swallow properly
- Patients who are status post neurological injury (e.g., stroke, traumatic brain injury, post-surgery) or radiation who have been noted to have swallowing difficulties
- Patients with congenital (i.e., present at birth) anatomic defects that affect swallowing where functional improvement is predicted (e.g., craniofacial impairments such as cleft palate and cleft lip)
- Patients with chronic conditions (e.g., Parkinson’s disease, amyotrophic lateral sclerosis, etc.) with new onset swallowing difficulties as evidenced by coughing and choking with meals, drooling, etc., may benefit from short-term swallowing therapy.
Exclusions:
- Blue Cross doesn’t cover swallowing/feeding therapy for food aversions because this is considered a behavioral problem. Treatment for behavioral problems isn’t covered under the speech/swallowing therapy benefit.
- Blue Cross doesn’t separately cover neuromuscular stimulation to the muscles of the throat to stimulate inactive swallowing muscles commonly found in patients with dysphagia (known as vital stimulation or VitalStim therapy) and similar non-specific electrical stimulation methods for the treatment of dysphagia unless included in a standard program of dysphagia rehabilitation.
- Blue Cross doesn’t cover deep pharyngeal neuromuscular stimulation, or DPNS.
- Any therapy involving digital stimulation of the mouth, tongue or pharynx in patients not having a specifically diagnosed neuromuscular disorder specifically and adversely effecting swallowing
- Swallowing therapy for deviant swallow or tongue thrust
|
95782, 95783, 95800, 95805- 95808,
95810, 95811, G0398, G0399
Not covered codes:
94762, 95801, E0445, G0400 |
Basic benefit and medical policy
Diagnosis of sleep disorders
Polysomnography, or PSG, is an attended (supervised) sleep study (sleep apnea test) performed in a hospital or freestanding sleep laboratory. The safety and effectiveness of PSG, including a split-night PSG, have been established. It may be considered a useful diagnostic option when indicated.
The safety and effectiveness of an unattended sleep study/sleep apnea test with a minimum of three recording channels (using, at a minimum, the following sensors: nasal pressure with chest and abdominal respiratory inductance plethysmography and oximetry; or using peripheral arterial tone, or PAT, with oximetry and actigraphy) in a home setting (home sleep apnea test) have been established. It may be considered a useful diagnostic option when indicated.
The safety and effectiveness of multiple sleep latency testing, or MSLT, have been established. It may be a useful tool in diagnosing narcolepsy.
Noninvasive pulse oximetry as a sole test (as an alternative to polysomnography or as a cardiorespiratory study for diagnosing sleep related breathing disorders) is considered experimental. Its effectiveness hasn’t been established.
Inclusionary and exclusionary criteria have been updated, effective July 1, 2023.
Inclusions:
Initial unattended (unsupervised) home sleep apnea test, or HSAT
This should be performed with a minimum of three recording channels (using, at a minimum, the following sensors: nasal pressure, chest and abdominal respiratory inductance plethysmography and oximetry; or using peripheral arterial tone, or PAT, with oximetry and actigraphy)
- Adults age 18 or older with high pretest probability for moderate to severe OSA (one of the following)
- Observed apneas during sleep
- A combination of at least two of the following:
- Excessive daytime sleepiness evidenced by an Epworth sleepiness >10, inappropriate daytime napping (e.g., during driving, conversation or eating), or sleepiness that interferes with daily activities and is not explained by other conditions
- Habitual snoring or gasping/choking episodes associated with awakenings
- Treatment-resistant hypertension (persistent hypertension in an individual taking three or more antihypertensive medications)
- Obesity, defined as a body mass index, or BMI, > 30 kg/m2 or neck circumference defined as >17 inches in men or >16 inches in women
- Craniofacial or upper airway soft tissue abnormalities
- Unexplained nocturia
- History of stroke (more than 30 days previously), transient ischemic attack, coronary artery disease or sustained supraventricular tachycardic or bradycardic arrhythmias in patients who meet one of the six criteria listed under “b” above.
- No exclusions/contraindications to a home sleep apnea test
Repeat unattended (unsupervised) follow-up home sleep apnea test
This should be performed with a minimum of three recording channels using, at a minimum, the following sensors: nasal pressure, chest and abdominal respiratory inductance plethysmography and oximetry; or using peripheral arterial tone, or PAT, with oximetry and actigraphy.
Inclusions (one of the following):
- To assess efficacy of surgery or oral appliances/devices
- To re-evaluate the diagnosis of OSA and need for continued continuous positive airway pressure, or CPAP, e.g., if there is a significant change in weight or change in symptoms suggesting that CPAP should be reiterated or possibly discontinued
Initial attended (supervised) sleep study performed in a sleep lab
Adults with suspected OSA:
Inclusions:
- Adults age 18 or older with a moderate to high pretest probability for OSA (one of the following)
- Observed apneas during sleep
- A combination of at least two of the following:
- Excessive daytime sleepiness evidenced by an Epworth sleepiness >10, inappropriate daytime napping (e.g., during driving, conversation or eating), or sleepiness that interferes with daily activities and isn’t explained by other conditions
- Habitual snoring or gasping/choking episodes associated with awakenings
- Treatment-resistant hypertension (persistent hypertension in an individual taking three or more antihypertensive medications)
- Obesity, defined as a BMI >30 kg/m2 or neck circumference > 17 inches in men or >16 inches in women
- Craniofacial or upper airway soft tissue abnormalities
- Unexplained nocturia
- History of stroke (more than 30 days previously), transient ischemic attack, coronary artery disease or sustained tachycardic or bradycardic arrhythmias in patients who meet one of the six criteria listed under “b” above.
- When unattended (unsupervised) home sleep apnea test is contraindicated (see exclusions/contraindications to unattended home sleep apnea test above)
- When the initial unattended (unsupervised) study was negative, inadequate, equivocal or non-diagnostic and clinical suspicion for OSA remains
Adults with suspected sleep disorders other than OSA:
An in-lab supervised sleep study may be considered when there is suspicion of any of the following:
- Central sleep apnea
- Narcolepsy
- Nocturnal seizures
- Parasomnia
- Idiopathic hypersomnia
- Periodic limb movement disorder, or PLMD, to support a suspicion of PLMD in this context, one of the following must be documented: Pregnancy, renal failure, iron deficiency anemia, peripheral neuropathy, use of antidepressant or antipsychotic medications, or continued hypersomnia and clinical symptoms of PLMD after sleep-disordered breathing is ruled out by home sleep apnea testing
- Nocturnal desaturation (due to severe COPD or certain restrictive thoracic disorders)
- Any of the following conditions (right heart failure, polycythemia, cardiac arrhythmias during sleep, or pulmonary hypertension) when the etiology is unclear
Children (younger than 18 years of age)
Inclusions:
- Pediatric individuals younger than 18 with a moderate to high probability of OSA (one of the following):
- Habitual snoring in association with one or more of the criteria below:
- Restless or disturbed sleep
- Behavioral disturbance or learning disorders including deterioration in academic performance, attention deficit disorder, hyperactivity
- Frequent awakenings
- Enuresis (bedwetting)
- Growth retardation or failure to thrive
- Excessive daytime somnolence or altered mental status not explained by other conditions
- Polycythemia not explained by other conditions
- Cor pulmonale not explained by other conditions
- Witnessed apnea with duration greater than two respiratory cycles
- Labored breathing during sleep
- Hypertrophy of the tonsils or adenoids in individuals at significant surgical risk such that the exclusion of OSA would allow avoidance of surgery
- Suspected congenital central alveolar hypoventilation syndrome or sleep-related hypoventilation due to neuromuscular disease or chest wall deformities
- Clinical evidence of a sleep-related breathing disorder in infants who have experienced an apparent life-threatening event
- For exclusion of OSA in an individual who has undergone adenotonsillectomy for suspected OSA more than eight weeks previously
- The initial study was inadequate, equivocal or non-diagnostic and the child’s parents or caregiver report that the breathing patterns observed at home were different from those during testing
Repeat attended (supervised) sleep study performed in a sleep lab
Adults aged 18 or older
Inclusions:
- Equipment failure or less than six hours of recording
- Initial PSG is negative and a clinical suspicion of OSA remains
- To initiate and titrate CPAP in adult individuals who have one of the following:
- An AHI or RDI of at least 15 events per hour
- An AHI or RDI of at least 5 events per hour in an individual with excessive daytime sleepiness or unexplained hypertension.
Note: A split-night study, in which moderate to severe OSA is documented during the first portion of the study using PSG, followed by CPAP during the second portion of the study, can eliminate the need for a second study to titrate CPAP
- To reevaluate the diagnosis of OSA and need for continued CPAP (e.g., if there is a significant change in weight or change in symptoms suggesting that CPAP should be retitrated or possibly discontinued.) Note: This statement doesn’t imply that supervised studies are needed routinely following unattended studies. This statement means a re-evaluation based on a substantial change in symptoms or in the clinical situation.
- To assess the efficacy of surgery (including adenotonsillectomy or upper airway) or oral appliances/devices
Children younger than 18 years of age
Inclusions:
- Initial PSG is negative and a clinical suspicion of OSA remains.
- Initial study was inadequate, equivocal or non-diagnostic and the child’s parents or caregiver report that the breathing patterns observed at home were different from those during testing.
- A patient with established OSA continues to exhibit persistent snoring or other symptoms of sleep-disordered breathing despite PAP adherence as defined by Centers for Medicare & Medicaid Services criteria (use of PAP for at least four hours per night on 70% of nights during a consecutive 30-day period).
- The patient has undergone adenotonsillectomy more than eight weeks previously for management of established OSA.
- To reevaluate the diagnosis of OSA and need for continued PAP if there is significant weight loss (defined as 10% of body weight) since the most recent sleep study
- To initiate or titrate CPAP or BPAP in a patient whose diagnostic study confirms that the patient is a candidate for positive airway pressure therapy and split-night study hasn’t been performed or was inadequate:
- In pediatric individuals, an AHI greater than 1.5 is considered abnormal, and an AHI of 10 or more may be considered severe.
- The initial sleep study has led to a diagnosis other than OSA and the repeat study is requested because of a change in clinical status or to assess efficacy after a change in therapy.
Multiple sleep latency testing, or MSLT
MSLT is considered experimental to diagnose obstructive sleep apnea, or OSA, except to exclude or confirm narcolepsy in the diagnostic workup of OSA syndrome.
Nonivasive pulse oximetry
The effectiveness of noninvasive pulse oximetry as a sole test (as an alternative to polysomnography or as a cardiorespiratory study for diagnosing sleep-related breathing disorders) is considered experimental. Its effectiveness hasn’t been established.
Exclusions:
Exclusions and contraindications to HSAT:
- Younger than 18 years
- Class III obesity, formerly referred to as morbid obesity, defined as a BMI >40 kg/m2 or the individual is 100 pounds over the ideal body weight for their height
- Obesity hypoventilation syndrome
- Narcolepsy
- Idiopathic hypersomnia
- Periodic limb movement disorder, or PLMD, when one of the following are present: Pregnancy, renal failure, iron deficiency anemia, peripheral neuropathy, use of antidepressant or antipsychotic medications, or continued hypersomnia and clinical symptoms of PLMD after sleep-disordered breathing is ruled out by home sleep apnea testing
- Central sleep disorder
- Parasomnias
- Nocturnal seizures
- REM behavior disorder
- Moderate or severe congestive heart failure – New York Heart Association (NYHA) class III or IV
- Congestive heart failure with a history of ventricular fibrillation or sustained ventricular tachycardia in an individual who does not have an implanted defibrillator
- Moderate or severe chronic pulmonary disease – forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) less than or equal to 0.7 and FEV1 less than 80% of predicted
- Documented neuromuscular disease (e.g., Parkinson’s, myotonic dystrophy, ALS)
- Severe insomnia or chronic opioid use
- Impairment that results in the inability to apply the home sleep apnea testing equipment
- Oxygen dependence
|
98975, 98976, 98977, 98980,
98981, 99453, 99454, 99457,
99458, 99091 |
Basic benefit and medical policy
Remote patient and therapeutic monitoring
The use of remote patient monitoring, or RPM, to collect physiological or psychological data in the medical management of patients is considered established when policy guidelines criteria are met.
The use of RTM in the medical management of an individual’s respiratory or musculoskeletal treatment plan is considered established when criteria are met.
Medical policy criteria have been updated, effective July 1, 2023.
RPM isn’t intended to be an ongoing modality; it’s intended to be an intervention in response to a complication, decompensation or instability of a medical condition. It may be used during the stabilization period, while a patient returns to the baseline of their condition or establishes a new baseline. Once baseline is achieved, RPM is no longer an integral part of a plan of care.
When Blue Cross Blue Shield of Michigan has an existing medical policy that is specific to the technology or device being considered for RPM, that policy supersedes the information in this policy.
Inclusions:
RPM is approved when both of the following are met:
- A physician or qualified health care practitioner has determined that the patient’s condition is one of the following:
- High risk for decompensation or complication that may lead to hospitalization or another acute intervention
- Requires monitoring for a current or new treatment plan
- There is an order written by a physician or qualified health care practitioner that specifies the medical condition and the length of time for RPM, up to 90 days.
Remote patient monitoring policy guidelines
RPM data
- Data may include common physiological parameters such as heart rate, blood pressure, temperature, respiratory rate, weight, oxygen saturation, peak flow, blood glucose levels, well-being information, etc.
RPM device guidance**
- The device used for data collection must be a medical device, as defined by the FDA.
- The device is non-invasive and has the potential to be connected to a wireless network through Bluetooth, Wi-Fi, or cellular connection.
- The device transmits a patient’s measurements directly to their health care provider or to a monitoring company affiliated with the health care provider.
- Some devices may have the potential to apply algorithms to the data, which result in notifications of parameters that are outside the ideal range for that patient.
- The device used must provide secure, HIPAA-compliant transmission of the data.
**Examples: Devices may include wearable, hand-held, stationary in-home units and digital interfaces. A device may be a clinical electronic thermometer, electrocardiograph, cardiac monitor, pulse oximeter, non-invasive blood pressure monitor, etc.
Services included in RPM
- Initial setup and patient instruction of the monitoring device
- RPM for up to 90 days
- Each 30-day billing cycle must include at least 16 days of monitoring.
- Remote patient monitoring should include daily monitoring or programmed alert transmissions.
- Remote patient monitoring programs can be offered by health plans, hospital systems, medical specialty groups or clinical practices.
- Reimbursement for remote patient monitoring is driven by current Blue Cross payment policy.
- Physician interpretation of the physiological or psychological data.
For RPM services beyond 90 days (all of the following)
- There is a physician or qualified health care practitioner order for the continuation of RPM.
- The medical record contains documentation that supports the medical necessity for continued RPM and reflects that the results of the monitoring are used in clinical decision-making and intervention.
- RPM (after the first 90 days) is billed with modifier KX (the provider attests that requirements specified in the medical policy have been met).
Note: Patients with complex, chronic conditions who are at elevated risk for intermittent exacerbations and poor long-term clinical outcomes may benefit from longer-term RPM within the context of a Provider-Delivered Care Management, health plan-administered care management program or an approved provider-organization or vendor-managed care management program.
RPM exclusions:
- RPM isn’t separately billable if performed during a 90-day global payment period (e.g., following surgery).
- The RPM device itself (including any additional apps, software, digital interfaces, etc.) isn’t covered.
Remote therapeutic monitoring, or RTM
Remote therapeutic monitoring services (e.g., musculoskeletal system status, respiratory system status, therapy adherence, therapy response) represent the review and monitoring of data related to signs, symptoms, and a function of therapeutic response. These data may represent objective device generated integrated data or subjective inputs reported by an individual. These data are reflective of therapeutic responses that provide a functionally integrative representative of patient status.
When Blue Cross Blue Shield of Michigan has an existing medical policy that is specific to the technology or device being considered for RTM, that policy supersedes the information in this policy.
Inclusions:
RTM is approved when there is an order written by a physician or qualified health care practitioner that specifies the medical condition and the length of time for RTM, up to 90 days.
Policy guidelines
RTM data
- Data may be self-reported by the individual or may be electronically captured by a device.
- Data is for a respiratory or musculoskeletal condition.
RTM device guidance (when a device is used)**
- The device used for data collection must be a medical device, as defined by the FDA.
- The device used must provide secure, HIPAA-compliant transmission of the data.
**Examples: Devices may include wearable, hand-held and digital interfaces.
Services included in RTM
- Initial set-up and patient instruction of the monitoring device.
- RTM for up to 90 days.
- Each 30-day billing cycle must include at least 16 days of monitoring.
- Reimbursement for remote therapeutic monitoring is driven by current Blue Cross payment policy.
For RTM services beyond 90 days (all the following)
- There is a physician or qualified health care practitioner order for the continuation of RTM.
- The medical record contains documentation that supports the medical necessity for continued RTM and reflects that the results of the monitoring are used in clinical decision-making and intervention.
- RTM after the first 90 days is billed with modifier KX (the provider attests that requirements specified in the medical policy have been met).
Note: Patients with complex, chronic conditions who are at elevated risk for intermittent exacerbations and poor long-term clinical outcomes may benefit from longer-term RPM within the context of a Provider-Delivered Care Management, health plan-administered care management program or an approved provider-organization or vendor-managed care management program.
RTM exclusions:
The RTM device itself (including any additional apps, software, digital interfaces, etc.) isn’t covered |
B4034, B4035, B4036, B4081- B4083,
B4087, B4088, B4102- B4104, B4149,
B4150, B4152- B4155, B4157- B4162,
B9002, B9998
Not covered code:
B4100 |
Basic benefit and medical policy
Enteral nutrition
The safety and effectiveness of enteral nutrition for individuals who meet the patient selection criteria have been established. It’s a useful therapeutic option when indicated.
Inclusionary and exclusionary criteria have been updated, effective July 1, 2023.
Requirements:
The patient must have an impairment that is long term or considered permanent. Coverage is possible for individuals with partial impairments, e.g., a patient with dysphagia who can swallow small amounts of food or a patient with Crohn’s disease who requires prolonged infusion of enteral nutrients to overcome problems with absorption.
Note: Permanence doesn’t require a determination that there is no possibility that the patient’s condition may improve sometime in the future. If the physician substantiates that a condition is of long and indefinite duration (ordinarily at least three months), the test of permanence may be met.
The medical record must document all information relevant to both of the following:
- The patient requiring the nutrition
- The nutritional prescription
Inclusions:
Enteral nutrition is established for individuals who require tube feedings to provide sufficient nutrients to maintain weight and strength commensurate with the individual’s overall health status due to the following conditions:
- A dysfunction of indefinite duration or disease of the structures that normally permit food to reach the small bowel
- A disease of the small bowel that impairs digestion and absorption of an oral diet
If enteral nutrition criteria are met, all tube feeding supplies are covered for the individual.
Note: When a feeding pump is requested, it must be supported by sufficient medical documentation to establish that the pump is medically necessary (e.g., gravity feeding is not satisfactory due to aspiration, diarrhea, dumping syndrome, etc.). Allowance is made for the simplest model that meets the medical needs of the patient as established by medical documentation.
Exclusions:
- Individuals with a functioning gastrointestinal tract whose need for enteral nutrition is due to reasons such as anorexia or nausea associated with mood disorder
- Individuals in whom adequate nutrition is possible by dietary adjustment or oral supplements
- Enteral nutrition products that are administered orally and related supplies
- Food thickeners, baby food, infant formulas and other regular grocery products aren’t covered even if they are given as enteral nutrition.
Notes:
- This policy doesn’t address infants (up to 12 months of age) who can’t tolerate cow milk formulas, soy formula, breast milk or hydrolyzed formulas who may require an elemental formula (e.g., Neocate®, Neocate® with DHA and ARA or EleCare).
- For individuals with inborn errors of metabolism who require specialized medical formula, refer to the policy “Medical Formula for Inborn Errors of Metabolism.”
|
C9399,
J3490,
J3590
|
Basic benefit and medical policy
Precedex (dexmedetomidine hydrochloride)
Effective Dec. 16, 2022, Precedex (dexmedetomidine hydrochloride) is covered for the following FDA-approved indications:
- Precedex (dexmedetomidine hydrochloride) is an alpha2-adrenergic receptor agonist indicated for the sedation of non-intubated pediatric patients aged 1 month to younger than 18 years prior to and during non-invasive procedures.
Dosage and administration:
For sedation of pediatric patients during non-invasive procedures: Patients 1 month to younger than 2 years old initiate at 1.5 mcg/kg over 10 minutes followed by a maintenance infusion of 1.5 mcg/kg/hour and titrated to achieve the desired clinical effect with the dosage ranging from 0.5 to 1.5 mcg/kg/hour; patients 2 to younger than 18 years old initiate at 2.0 mcg/kg over 10 minutes followed by a maintenance infusion of 1.5 mcg/kg/hour and titrated to achieve the desired clinical effect with the dosage ranging from 0.5 to 1.5 mcg/kg/hour. |
E0486
Experimental/not covered codes:
A7047, E0485, E1399,** K1001,
K1028, K1029 **Not otherwise classified procedure |
Basic benefit and medical policy
Medical management of obstructive sleep apnea through oral appliances and novel therapies
The safety and effectiveness of oral appliances to reduce upper airway collapsibility in the treatment of obstructive sleep apnea, or OSA, have been established. An oral appliance may be considered a useful therapeutic option when indicated.
Palate and mandible expansion devices are considered experimental for the treatment of OSA. There is insufficient evidence in the current medical literature to support their efficacy and use in clinical practice.
Nasal expiratory positive airway pressure, or nasal EPAP, an inserted nasal valve, used for the treatment of OSA is considered experimental. There is insufficient evidence in the current medical literature to support its efficacy and use in clinical practice.
Oral pressure therapy for the treatment of OSA is considered experimental. There is insufficient medical literature found to support its efficacy.
The use of sleep positioning trainers with vibration, such as the NightBalance Lunoa SPT system, for the treatment of positional OSA are considered experimental. They haven’t been proven to be more effective than standard care.
The use of an abbreviated daytime sleep session for acclimation to CPAP (PAP-NAP) is considered experimental.
The use of daytime electrical stimulation of the tongue is considered experimental for the treatment of OSA.
Inclusions:
Intraoral appliances (tongue-retaining devices or mandibular advancing/positioning devices (all the following):
- Diagnosis of OSA, as defined by one of the following:
- An AHI, RDI or REI of at least 15 events per hour
- An AHI, RDI or REI of at least five events per hour in an individual with one or more signs or symptoms associated with OSA (e.g., excessive daytime sleepiness, impaired cognition, mood disorders, insomnia, hypertension, ischemic heart disease, or history of stroke)
- A trial of CPAP has failed, isn’t tolerated by the individual or is contraindicated.
- The device is prescribed by the treating physician.
- The device is custom fitted by a dentist (preferably a dentist with certification/additional training in dental sleep medicine).
- There is a dental evaluation that documents absence of both temporomandibular dysfunction and periodontal disease.
Impressions, models, fabrication, materials, insertion/fitting, training, subsequent adjustments/modifications of the appliance, repairs and ancillary appliances are included with the OSA appliance and aren’t separately billable for the first 90 days after provision of the oral appliance.
Replacement of an oral appliance may be considered at the end of the five-year reasonable useful lifetime or prior if there’s a change in the individual’s condition.
Oral appliances for OSA (e.g., tongue-retaining devices or mandibular orthopedic positioning devices) may be considered established in adult individuals with clinically significant OSA. (Verify coverage of intraoral appliances under the DME benefit.)
Definition of an oral appliance for OSA
- A custom-fabricated appliance, using digital or physical impressions and models of an individual’s oral structures and physical needs
- Not a prefabricated item that is modified. Prefabricated components may be included in the final appliance.
- Includes all appliances, including titration appliances
- Made of biocompatible materials
- Engages the maxillary and mandibular arches
- Includes a mechanism that advances the mandible in increments of 1 mm or less with a protrusive adjustment range of at least 5 mm. This mechanism may or may not include fixed mechanical hinges or metallic materials.
- Reversal of the advancement is possible
- The protrusive setting must be verifiable
An appropriate oral appliance will allow for optimal protrusion of the mandible (e.g., less than 5 mm) to produce the desired relative opening of the airway, without contributing to an increased risk of temporal mandibular joint dysfunction.
PAP therapies, i.e., continuous positive airway pressure, or CPAP; automatic positive airway pressure, or APAP; bilevel positive airway pressure, or BiPAP, and variable positive airway pressure, or VPAP, may be considered medically necessary for the management of OSA, central sleep apnea or mixed apnea. These devices for treatment are addressed in the policy titled “Positive Pressure Airway Devices.”
.
Exclusions:
- The use of intraoral appliances that don’t meet the above criteria are considered experimental for the treatment of OSA.
- Prefabricated (not custom-fit) devices (e.g., sport mouth guards, mouth guards that can be purchased in a retail store or pharmacy)
- Screening tests (e.g., questionnaire, pulse oximetry, rhinometry and laryngometry, etc.) performed by a dentist
The use of an abbreviated daytime sleep session for acclimation to CPAP (PAP-NAP) is considered experimental.
The use of daytime electrical stimulation of the tongue is considered experimental for the treatment of OSA.
Palate and mandible expansion devices for the treatment of OSA are considered experimental.
Nasal expiratory positive airway pressure and oral pressure therapy devices are considered experimental.
The use of sleep positioning trainers with vibration, such as the NightBalance Lunoa SPT system, for the treatment of positional OSA is considered experimental. |
J0714 |
Basic benefit and medical policy Avycaz (ceftazidime and avibactam)
Avycaz (ceftazidime and avibactam) is considered established when criteria are met, effective Dec. 20, 2022. Avycaz (ceftazidime and avibactam) has been approved for the following updated indications:
Avycaz is a combination of ceftazidime, a cephalosporin, and avibactam, a beta-lactamase inhibitor, indicated for the treatment of infections caused by designated susceptible Gram-negative microorganisms in adult and pediatric patients age 3 months and older.
Dosage and administration:
Dosage of Avycaz in pediatric patients aged 2 years to less than 18 years with estimated glomerular filtration rate, or eGFR, greater than 50 mL/min/1.73 m2 and 3 months to less than 2 years without renal impairment
infection: cIAI, cUTI including pyelonephritis and HABP/VABP
Age range: 2 years to less than 18 years
Dose: Avycaz 62.5 mg/kg to a maximum of 2.5 grams (ceftazidime 50 mg/kg and avibactam 12.5 mg/kg to a maximum dose of ceftazidime 2 grams and avibactam 0.5 grams)
Age range: 6 months to less than 2 years
Dose: Avycaz 62.5 mg/kg (ceftazidime 50 mg/kg and avibactam 12.5 mg)
Age range: 3 months to less than 6 months
Dose: Avycaz 50 mg/kg (ceftazidime 40 mg/kg and avibactam 10 mg/kg)
Infusion time/frequency:
Two hours/every eight hours
|
J1305 |
Basic benefit and medical policy
Evkeeza (evinacumab-dgnb)
Effective March 21, 2023, Evkeeza (evinacumab-dgnb) is covered for the following FDA-approved indications:
Evkeeza is an angiopoietin-like 3, or ANGPTL3, inhibitor indicated as an adjunct to other low-density lipoprotein-cholesterol, or LDL-C, lowering therapies for the treatment of adult and pediatric patients aged 5 years and older with homozygous familial hypercholesterolemia, or HoFH. |
J3490
J3590 |
Basic benefit and medical policy
Lamzede (velmanase alfa-tycv)
Effective Feb. 16, 2023, Lamzede (velmanase alfa-tycv) is covered for the following FDA-approved indications:
- Lamzede is recombinant human lysosomal alpha-mannosidase indicated for the treatment of non-central nervous system manifestations of alpha-mannosidosis in adult and pediatric patients.
Dosage and administration:
- For females of reproductive potential, verify that the patient isn’t pregnant prior to initiating treatment.
- Consider pretreating with antihistamines, antipyretics or corticosteroids prior to Lamzede administration.
- Recommended Lamzede dosage is 1 mg/kg (actual body weight) administered once every week as an intravenous infusion.
Dosage forms and strengths:
For injection: 10 mg of velmanase alfa-tycv as a lyophilized powder in a single-dose vial for reconstitution.
Lamzede (velmanase alfa-tycv) isn’t a benefit for URMBT. |
J3490
J3590 |
Basic benefit and medical policy
Leqembi (lecanemab-irmb)
Effective Jan. 6, 2023, Leqembi (lecanemab-irmb) is considered established when criteria are met.
Leqembi (lecanemab-irmb) is an amyloid beta-directed antibody indicated for the treatment of Alzheimer’s disease. Treatment with Leqembi (lecanemab-irmb) should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials. There are no safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied. This indication is approved under accelerated approval based on reduction in amyloid beta plaques observed in patients treated with Leqembi (lecanemab-irmb). Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial.
Dosage and administration:
- Confirm the presence of amyloid beta pathology before initiating treatment.
- The recommended dosage is 10 mg/kg that must be diluted and then administered as an intravenous infusion over approximately one hour, once every two weeks.
- Obtain a recent (within one year) brain MRI before initiating treatment to evaluate for pre-existing amyloid related imaging abnormalities, or ARIA.
- Obtain an MRI before the fifth, seventh and 14th infusions. If radiographically observed ARIA occurs, treatment recommendations are based on type, severity and presence of symptoms.
- Dilution in 250 mL of 0.9% sodium chloride injection, USP, is required before administration.
- Administer as an intravenous infusion over approximately one hour via a terminal low-protein binding 0.2 micron in-line filter.
Dosage forms and strengths:
Injection:
- 500 mg/5 mL (100 mg/mL) solution in a single-dose vial
- 200 mg/2 mL (100 mg/mL) solution in a single-dose vial
Leqembi (lecanemab-irmb) isn’t covered for URMBT.
|
J3490
J3590 |
Basic benefit and medical policy
Rykindo (risperidone injection)
Rykindo (risperidone injection) is considered established when criteria are met, effective Jan. 13, 2023.
Rykindo is an atypical antipsychotic indicated:
- For the treatment of schizophrenia in adults.
- As monotherapy or as adjunctive therapy to lithium or valproate for the maintenance treatment of bipolar I disorder in adults.
Dosage and administration:
- Establish tolerability with oral risperidone before initiating treatment with Rykindo.
- Administer Rykindo by intramuscular, or IM, injection in the gluteal muscle by a health care provider. Don’t administer by any other route.
- Recommended dosage of Rykindo is 25 mg intramuscular every two weeks. Patients not responding to 25 mg may benefit from 37.5 mg or 50 mg. Dosage titration shouldn’t be made more frequently than every four weeks. The maximum recommended dosage should not exceed 50 mg every two weeks.
- Administer the first dose of Rykindo along with seven days of oral risperidone.
- Renal or hepatic impairment: Titrate with oral risperidone up to at least 2 mg before initiating treatment with Rykindo. A starting dose of 12.5 mg may be appropriate for some patients.
Dosage forms and strengths:
For extended-release injectable suspension: 12.5 mg, 25 mg, 37.5 mg, and 50 mg.
Rykindo (risperidone injection) isn’t a benefit for URMBT. |
J9022 |
Basic benefit and medical policy
Tecentriq (atezolizumab)
Effective Dec. 9, 2022, Tecentriq (atezolizumab) is payable for the following updated FDA-approved indications:
- Tecentriq is a programmed death-ligand 1, or PD-L1, blocking antibody indicated for alveolar soft part sarcoma, or ASPS, for the treatment of adult and pediatric patients 2 years and older with unresectable or metastatic ASPS.
Dosing information:
ASPS
- Adults: Administer Tecentriq as 840 mg every two weeks, 1,200 mg every three weeks or 1,680 mg every four weeks.
- Pediatric patients 2 years of age and older: Administer 15 mg/kg (up to a maximum of 1,200 mg), every three weeks.
|
J9271 |
Basic benefit and medical policy
Keytruda (pembrolizumab)
Blue Cross Blue Shield of Michigan has approved payment for the off-label use of Keytruda (pembrolizumab) for the treatment of malignant neoplasm of the retroperitoneum.
URMBT is excluded from this change. |
J9272 |
Basic benefit and medical policy
Jemperli (dostarlimab-gxly)
Effective Feb. 9, 2023, Jemperli (dostarlimab-gxly) is covered for the following FDA-approved indications:
Jemperli (dostarlimab-gxly) is indicated for the following usage under the indication for endometrial cancer, as determined by an FDA-approved test, that has progressed on or following prior treatment with a platinum-containing regimen in any setting, and the patient isn’t a candidate for curative surgery or radiation. |
J9304 |
Basic benefit and medical policy
Pemfexy (pemetrexed)
Effective Dec. 14, 2022, Pemfexy (pemetrexed) is payable for the following FDA-approved usage related to metastatic non-squamous non-small cell lung cancer, or NSCLC:
In combination with pembrolizumab and platinum chemotherapy, for the initial treatment of patients with metastatic non-squamous NSCLC, with no estimated glomerular filtration rate, or eGFR, or anaplastic lymphoma kinase, or ALK, genomic tumor aberrations |
J9317 |
Basic benefit and medical policy
Trodelvy (sacituzumab govitecan-hziy)
Trodelvy (sacituzumab govitecan-hziy) is considered established when criteria are met, effective Feb. 3, 2023.
Trodelvy (sacituzumab govitecan-hziy) has been approved for the following updated indications:
Unresectable locally advanced or metastatic hormone receptor-positive, human epidermal growth factor receptor 2-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting |
S2202, 36465, 36466, 36470, 36471,
36475, 36476, 36478, 36479, 36482,
36483, 37500, 37700, 37718, 37722,
37735, 37760, 37761, 37765, 37766,
37780, 37785, 76942
Not covered:
36468, 36473, 36474, 0524T, 37799 |
Basic benefit and medical policy
Treatment of varicose veins/venous insufficiency
The treatment of the great or small saphenous veins by surgery (ligation and stripping), endovenous thermal ablation (radiofrequency or laser), microfoam sclerotherapy or cyanoacrylate adhesive is considered established when criteria are met.
The treatment of accessory saphenous veins by surgery (ligation and stripping), endovenous radiofrequency or laser ablation, microfoam sclerotherapy or cyanoacrylate adhesive is considered established when criteria have been met.
The treatment of symptomatic varicose tributaries is considered established when criteria are met.
Surgical ligation (including subfascial endoscopic perforator surgery), endovenous radiofrequency or laser ablation of incompetent perforator veins is considered established as a treatment of leg ulcers associated with chronic venous insufficiency when criteria are met.
Endovenous ablation of varicose veins by mechanochemical (ClariVein®) is experimental. This procedure hasn’t been scientifically demonstrated to be as safe and effective as conventional treatment.
Endovenous cryoablation of any vein is experimental. This procedure hasn’t been scientifically demonstrated to be as safe and effective as conventional treatment.
Inclusionary and exclusionary criteria have been updated, effective July 1, 2023.
Inclusions:
Great or small saphenous veins
Treatment of the great or small saphenous veins by surgery (ligation and stripping), endovenous thermal ablation (radiofrequency or laser), microfoam sclerotherapy or cyanoacrylate adhesive criteria (must meet both):
- There is demonstrated saphenous reflux and CEAP, known as Clinical, Etiology, Anatomy, Pathophysiology, class C2 or greater.
- There is documentation of one or more of the following indications:
- Ulceration secondary to venous stasis
- Recurrent superficial thrombophlebitis
- Hemorrhage or recurrent bleeding episodes from a ruptured superficial varicosity
- Persistent pain, swelling, itching, burning or other symptoms are associated with saphenous reflux and the symptoms significantly interfere with activities of daily living and conservative management including compression therapy for at least three months hasn’t improved the symptoms.
- Conservative management must include a trial of compression therapy garments.
- Medical reason for compression therapy exemption is documented (e.g., existing chronic limb ischemia, severe musculoskeletal disability, morbid obesity, unusual leg anatomy) .
Accessory saphenous veins
Treatment of accessory saphenous veins by surgery (ligation and stripping), endovenous radiofrequency or laser ablation, microfoam sclerotherapy or cyanoacrylate adhesive criteria (must meet all):
- Incompetence of the accessory saphenous vein is isolated.
- There is demonstrated accessory saphenous reflux.
- There is documentation of one or more of the following indications:
- Ulceration secondary to venous stasis
- Recurrent superficial thrombophlebitis
- Hemorrhage or recurrent bleeding episodes from a ruptured superficial varicosity
- Persistent pain, swelling, itching, burning, or other symptoms are associated with saphenous reflux, and the symptoms significantly interfere with activities of daily living, and conservative management including compression therapy for at least three months has not improved the symptoms.
- Conservative management must include a trial of compression therapy garments,
- Medical reason for compression therapy exemption is documented (e.g., existing chronic limb ischemia, severe musculoskeletal disability, morbid obesity, unusual leg anatomy)
Concurrent treatment of the accessory saphenous veins along with the great or small saphenous veins is considered established when criteria are met for each vein and there is documentation of anatomy showing that the accessory saphenous vein discharged directly into the common femoral vein.
Note: Radiofrequency or laser treatment of varicose veins will be covered for two treatments per lower extremity per patient per lifetime. Any additional claims should be submitted using an NOC code to facilitate evaluation for reimbursement. An unusual situation, such as a demonstrated recanalized vessel, would be considered for reimbursement on an individual case-by-case basis.
Symptomatic varicose tributaries
The following treatments are considered established in the treatment of symptomatic varicose tributaries** of the saphenous veins (none of these techniques has been shown to be superior to another):
- Stab avulsion
- Hook phlebectomy
- Sclerotherapy
- Transilluminated powered phlebectomy
**When performed either at the same time or following prior treatment (surgical, radiofrequency or laser).
Perforator veins
Surgical ligation (including subfascial endoscopic perforator surgery), endovenous radiofrequency or laser ablation of incompetent perforator veins as a treatment of leg ulcers associated with chronic venous insufficiency criteria (must meet all):
- There is demonstrated perforator reflux.
- The superficial saphenous veins (great, small, or accessory saphenous and symptomatic varicose tributaries) have been previously eliminated.
- Ulcers have not resolved following combined superficial vein treatment and compression therapy for at least three months.
- The venous insufficiency isn’t secondary to deep venous thromboembolism.
Telangiectasia
Treatment of telangiectasia, such as spider veins, angiomata and hemangiomata, is considered cosmetic and not covered.
Exclusions:
Techniques for conditions not specifically listed above are considered experimental, including, but not limited to:
- Sclerotherapy techniques, other than microfoam sclerotherapy, of great, small or accessory saphenous veins
- Sclerotherapy of perforator veins
- Sclerotherapy of isolated tributary veins without prior or concurrent treatment of saphenous veins
- Stab avulsion, hook phlebectomy or transilluminated powered phlebectomy of perforator, great or small saphenous, or accessory saphenous veins
- Endovenous radiofrequency or laser ablation of tributary veins
- Mechanochemical ablation of any vein
- Endovenous cryoablation of any vein
- Ligation or ablation of incompetent perforator veins performed concurrently with superficial venous surgery isn’t covered.
|
None of the information included in this billing chart is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
|



