|
July 2021

Timely clinical information is key to receiving faster responses on authorization requests
We’ve received complaints from members that it sometimes takes too long for their services to be authorized by Blue Cross Blue Shield of Michigan, Blue Care Network or by a company we’re working with to provide utilization management decisions for certain procedures.
Keep in mind that the National Committee for Quality Assurance and the Centers for Medicare & Medicaid Services require Blue Cross and BCN to respond to authorization requests within certain time frames.
We’re working to improve our response times and ask for your help to prevent an authorization request from being delayed or denied. It’s important that health care providers respond quickly to requests for documentation to prevent a delay of necessary or urgent medical services for members. We require clinical information for authorization requests to ensure that we make a timely and appropriate decision. Companies we work with to manage certain procedures may also ask providers for clinical information to support your requests.
It’s important that you provide requested clinical information and other documentation within the designated time frame indicated in the correspondence from Blue Cross, BCN or the company we’re working with to handle utilization requests.
Clinical information includes relevant information regarding a member’s:
- Health history
- Physical assessment
- Test results
- Consultations
- Previous treatment
We recommend that you’re prepared with clinical information at the time you submit your request in the event you’re asked to provide it. Much of the follow‑up information that we request is found on the questionnaires on erefferals.bcbsm.com. You can find preview questionnaires with links to related authorization criteria or medical policies on these webpages:
Companies we work with that manage certain utilization management programs usually have their own versions of these questionnaires.
The most efficient way to provide clinical information for programs managed by Blue Cross and BCN is through the e‑referral system. Use the Case Communication section to document how the patient meets clinical criteria.
For more information about utilization review, refer to our provider manuals. Here’s how to find them:
- Log in as a provider at bcbsm.com.
- Click on Provider Manuals on the lower right side of the screen.
- Select the manual you want to review.
The Utilization Management chapter of the BCN Provider Manual is also posted on ereferrals.bcbsm.com. You can get there without logging in. Click on Provider Manual Chapters under BCN Authorizations/Referrals in the left‑hand column.
Here’s how to choose the correct servicing provider in e‑referral to avoid denied claims
To avoid any problems when submitting authorizations and referrals in e‑referral, we’re clarifying the steps for choosing the correct servicing provider.
Keep in mind that the provider you’re looking for may be listed multiple times. Here’s how to make sure you’re choosing the correct listing:
- When your servicing provider results are returned, select the listing based on where the member is going to see the provider.
- If the provider has several listings with the same address, select the listing that also shows a group affiliation. If there are multiple group affiliations listed, make sure to choose the correct one.
- Not all provider addresses will be considered in network. If you select a listing that shows the provider is out of network (“Out” in the Network column), you’ll have to go through an out‑of‑network review.
This information can be found in the following sections of the e‑referral user guides:
- e-referral User Guide
- “Submit a Global Referral”
- “Submit a Referral”
- “Submit an Inpatient Authorization”
- “Submit an Outpatient Authorization”
- Behavioral Health e-referral User Guide
- “Submitting Higher Levels of Care Inpatient Authorizations”
- “Submitting Higher Levels of Care Outpatient Authorizations”
- “Submitting an Electroconvulsive Therapy Authorization”
- “Submitting a Transcranial Magnetic Stimulation Authorization”
- “Submitting a Neurofeedback Authorization”
It can also be found in the e-referral Quick Guide under the “Select provider/patient” section.
2021 HCPCS COVID‑19 update
The Centers for Medicare & Medicaid Services has added two COVID‑19 codes and deleted two as part of its quarterly Health Care Procedure Coding System updates. The codes, effective dates and Blue Cross Blue Shield of Michigan’s coverage decisions are below.
| Code |
Change |
Coverage comments |
Effective date |
| M0239 |
Deleted |
|
April 16, 2021 |
| M0244 |
Added |
Covered |
May 6, 2021 |
| M0246 |
Added |
Covered |
May 6, 2021 |
| Q0239 |
Deleted |
|
April 16, 2021 |
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
New Directions offers free educational series on applied behavioral analysis
Action item
Register for one or more of New Directions’ free webinars on best practices in applied behavioral analysis.
New Directions, Blue Cross Blue Shield of Michigan’s behavioral health manager for most of our commercial plans, will be offering a series of free educational webinars on applied behavioral analysis. The series will be led by board‑certified behavior analysts. Though this series is designed for ABA providers, all providers are welcome to attend. These monthly education sessions will focus on best practices in ABA and ethical considerations, including:
- Autism and mental health comorbidities
- Parent training, education and support
- Transition planning
- Coordination of care
- Informed consent
- Client dignity
- Child abuse reporting and maintaining therapeutic alliance
- Suicidality and autism spectrum disorder
- “Gold standard” documentation
Attendees will receive a continuing education unit after participation. New Directions is accredited as a CEU provider by the Behavioral Analysis Certification Board. Webinars will follow all BACB requirements.
Click here** to register for the upcoming webinars.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Billing chart: Blues highlight medical, benefit policy changes
You’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart.
This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures.
You will also see that descriptions for the codes are no longer included. This is a result of recent negotiations with the AMA on use of the codes.
We will publish information about new BCBS groups or changes to group benefits under the Group Benefit Changes heading.
For more detailed descriptions of the BCBSM policies for these procedures, please check under the Medical/Payment Policy tab in Explainer on web‑DENIS. To access this online information:
- Log in to web‑DENIS.
- Click on BCBSM Provider Publications & Resources.
- Click on Benefit Policy for a Code.
- Click on Topic.
- Under Topic Criteria, click on the drop‑down arrow next to Choose Identifier Type and then click on HCPCS Code.
- Enter the procedure code.
- Click on Finish.
- Click on Search.
| Code* |
BCBSM changes to:
Basic Benefit and Medical Policy, Group
Variations Payment Policy, Guidelines
|
| NEW PAYABLE PROCEDURES |
69705, 69706
Experimental:
0583T
|
Basic benefit and medical policy
Balloon dilation of the eustachian tube
Effective March 1, 2021, balloon dilation of the eustachian tube for treatment of chronic obstructive eustachian tube dysfunction may be considered established under all the following conditions:
- Adults (aged 18 and older) with symptoms of obstructive eustachian tube dysfunction (aural fullness, aural pressure, otalgia or hearing loss) for 12 months or longer in one or both ears that significantly affects quality of life or functional health status.
- Aural fullness and pressure present
- The patient has undergone a comprehensive diagnostic assessment, including patient-reported questionnaires, history and physical exam, tympanometry if the tympanic membrane is intact, nasal endoscopy and comprehensive audiometry, with the following findings:
- Abnormal tympanogram (Type B or C)
- Abnormal tympanic membrane (retracted membrane, effusion, perforation or any other abnormality identified on exam)
- Failure to respond to appropriate medical management of potential co‑occurring conditions, if any, such as allergic rhinitis, rhinosinusitis and laryngopharyngeal reflux, including four to six weeks of a nasal steroid spray, if indicated.
- Other causes of aural fullness, such as temporomandibular joint disorders, extrinsic obstruction of the eustachian tube, superior semicircular canal dehiscence and endolymphatic hydrops have been ruled out.
- If the patient had a history of tympanostomy tube placement, symptoms of obstructive eustachian tube dysfunction should have improved while tubes were patent.
- The patient doesn’t have patulous eustachian tube dysfunction or another contraindication to the procedure (see policy guidelines).
- The patient’s eustachian tube dysfunction has been shown to be reversible (see policy guidelines).
- Symptoms are continuous rather than episodic (e.g., symptoms occur only in response to barochallenge, such as pressure changes while flying).
- The patient hasn’t had a previous BDET procedure.
Balloon dilation of the eustachian tube is considered experimental if the above criteria aren’t met.
Policy guidelines:
Symptoms of obstructive eustachian tube dysfunction may include aural fullness, aural pressure, otalgia and hearing loss. Nearly all patients will have aural fullness and aural pressure. Many patients will have otalgia, but hearing loss may not be present in all patients (e.g., patients with Type C tympanograms).
Contraindications to balloon dilation of the eustachian tube
The following patients shouldn’t be considered for balloon dilation of the eustachian tube:
- Patients with patulous eustachian tube dysfunction
- A diagnosis of patulous ETD is suggested by symptoms of autophony of voice, audible respirations, pulsatile tinnitus or aural fullness
- Patients with extrinsic reversible or irreversible causes of eustachian tube dysfunction, including but not limited to:
- Craniofacial syndromes, including cleft palate spectrum
- Neoplasms causing extrinsic obstruction of the eustachian tube
- History of radiation therapy to the nasopharynx
- Enlarged adenoid pads
- Nasopharyngeal mass
- Neuromuscular disorders that lead to hypotonia/ineffective eustachian tube dynamic opening
- Systemic mucosal or autoimmune inflammatory disease affecting the mucosa of the nasopharynx and eustachian tube (e.g., Samter’s triad, Wegener’s disease, mucosal pemphigus) that is ongoing or active (i.e., not in remission)
- Patients with aural fullness but normal exam and tympanogram
- Patients with chronic and severe atelectatic ears
Reversibility of eustachian tube dysfunction
Reversibility of eustachian tube dysfunction can be demonstrated by several means, including any of the following:
- The patient states that they are able to relieve the pressure by performing a Valsalva maneuver to “pop” their ears.
- Performing a Valsalva maneuver produces temporary improvement of the patient’s tympanogram to Type A tympanogram.
- Performing a Valsalva maneuver causes the member’s middle ear to aerate, which is indicated by the provider visualizing lateral movement of the tympanic membrane on otoscopy.
Balloon dilation of the eustachian tube used in combination with other procedures
- Patients undergoing BDET concurrent with sinus ostial dilation should meet the same diagnostic criteria for BDET as those undergoing BDET alone.
- Patients with a middle ear effusion at the time of BDET may benefit from concurrent myringotomy with or without tympanostomy tube placement.
|
| UPDATES TO PAYABLE PROCEDURES |
J3490
J3590
|
Basic benefit and medical policy
Margenza (margetuximab‑cmkb)
Margenza (margetuximab‑cmkb) is payable for its FDA‑approved indications when billed with procedure code J3490 or J3590, effective Dec. 17, 2020. Margenza (margetuximab‑cmkb) should be reported with procedure code J3490 or J3590 and the appropriate national drug code until a permanent code is established.
URMBT groups are excluded from coverage of this drug.
Margenza (margetuximab‑cmkb) is a HER2/neu receptor antagonist indicated, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2‑positive breast cancer who have received two or more prior anti‑HER2 regimens, at least one of which was for metastatic disease.
Dosage and administration:
Administer Margenza (margetuximab‑cmkb) as an intravenous infusion at 15 mg/kg over 120 minutes for the initial dose, then over a minimum of 30 minutes every three weeks for all subsequent doses.
Dosage forms and strengths:
Injection: 250 mg/10 mL (25 mg/mL) in a single‑dose vial |
J9299 |
Basic benefit and medical policy
Opdivo (nivolumab)
Opdivo (nivolumab), procedure code J9299, is payable for its updated FDA‑approved indications.
Indications have been updated to include treatment of adult patients with unresectable malignant pleural mesothelioma, as first‑line treatment in combination with ipilimumab. |
| POLICY CLARIFICATIONS |
J0585 |
Basic benefit and medical policy
Botox (Onabotulinum‑toxinA)
Effective Feb. 9, 2021, Botox (Onabotulinum‑toxinA) is covered for the following FDA‑approved indications:
Botox is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for:
- Treatment of neurogenic detrusor overactivity, or NDO, in pediatric patients aged 5 years and older who have an inadequate response to or are intolerant of anticholinergic medication.
Dosing information:
- Follow indication‑specific dosage and administration recommendations. In a three‑month interval, don’t exceed a total dose of:
- Adults: 400 units
- Pediatrics: The lesser of 10 units/kg or 340 units
- Adult detrusor overactivity associated with a neurologic condition: Recommended total dose 200 units, as 1 mL (~6.7 Units) injections across 30 sites into the detrusor
- Pediatric detrusor overactivity associated with a neurologic condition: 0.5 mL injections across 20 sites into the detrusor:
- Greater than or equal to 34 kg: Recommended total dose is 200 units
-
Less than 34 kg: Recommended total dose is 6 units/kg
|
J3357 |
Basic benefit and medical policy
Stelara (ustekinumab)
Stelara (ustekinumab) is payable for the following updated FDA indications:
Pediatric patients age 6 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy
Psoriasis pediatric patients (aged 6 to 17) subcutaneous recommended dosage
Weight‑based dosing is recommended at the initial dose, four weeks later, then every 12 weeks thereafter.
| Weight range (kilogram) |
Dosage regimen |
| Less than 60 kg |
0.75 mg/kg |
| 60 kg to 100 kg |
45 mg |
| Greater than 100 kg |
90 mg |
|
J3490
J3590
|
Basic benefit and medical policy
Amondys 45 (casimersen)
Amondys 45 (casimersen) is considered experimental for all indications. Therefore, the service isn’t payable, effective Feb. 25, 2021. |
J3490
J3590
J9999
|
Basic benefit and medical policy
Breyanzi (lisocabtagene maraleucel)
Effective Feb. 5, 2021, Breyanzi (lisocabtagene maraleucel) is covered for the following FDA‑approved indications:
Breyanzi (lisocabtagene maraleucel) is a CD19‑directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory large B‑cell lymphoma after two or more lines of systemic therapy, including diffuse large B‑cell lymphoma, or DLBCL, not otherwise specified (including DLBCL arising from indolent lymphoma), high‑grade B‑cell lymphoma, primary mediastinal large B‑cell lymphoma and follicular lymphoma grade 3B.
Limitation of use:
- Breyanzi isn’t indicated for the treatment of patients with primary central nervous system lymphoma.
Dosage and administration:
- For autologous use only. For intravenous use only.
- A single dose of Breyanzi contains 50 to 110 × 106 CAR‑positive viable T cells (consisting of 1:1 CAR‑positive viable T cells of the CD8 and CD4 components), with each component supplied separately in one to four single‑dose vials.
Dosage forms and strengths:
- The dose is 50 to 110 × 106 CAR-positive viable T cells (consisting of CD8 and CD4 components). Administer Breyanzi (lisocabtagene maraleucel) in a certified health care facility.
- Each mL contains 1.5 × 106 to 70 × 106 CAR-positive viable T cells.
This drug isn’t a benefit for URMBT.
|
J3490
J3590
|
Basic benefit and medical policy
Cosela (trilaciclib dihydrochloride)
Effective Feb. 12, 2021, Cosela (trilaciclib dihydrochloride) is covered for the following FDA‑approved indications:
Indications and usage:
Cosela is a kinase inhibitor used to decrease the incidence of chemotherapy‑induced myelosuppression in adult patients when administered prior to a platinum/etoposide‑containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer.
Dosage and administration:
Recommended dosage: The recommended dose of Cosela is 240 mg/m2 per dose. Administer as a 30‑minute intravenous infusion completed within four hours prior to the start of chemotherapy on each day chemotherapy is administered.
Dosage forms and strengths:
For injection: 300 mg of trilaciclib as a lyophilized cake in a single‑dose vial
This drug isn’t a benefit for URMBT and MPSERS. |
J3490
J3590
|
Basic benefit and medical policy
Evkeeza (evinacumab‑dgnb)
Effective Feb. 11, 2021, Evkeeza (evinacumab‑dgnb) is covered for the following FDA‑approved indications:
Evkeeza is an ANGPTL3 (angiopoietin-like 3) inhibitor indicated as an adjunct to other low‑density lipoprotein‑cholesterol, or LDL‑C, lowering therapies for the treatment of adult and pediatric patients, age 12 and older, with homozygous familial hypercholesterolemia, or HoFH. |
J3490
J3590 |
Basic benefit and medical policy
Pepaxto (melphalan flufenamide)
Effective Feb. 26, 2021, Pepaxto (melphalan flufenamide) is covered for the following FDA‑approved indications:
Pepaxto is an alkylating drug indicated, in combination with dexamethasone, for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy and whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent and one CD38‑directed monoclonal antibody. This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitations of use:
Pepaxto isn’t indicated or recommended for use as a conditioning regimen for transplant outside of controlled clinical trials.
Dosage and administration:
The recommended dosage of Pepaxto is 40 mg intravenously over 30 minutes on Day 1 of each 28‑day treatment cycle, in combination with dexamethasone.
Dosage forms and strengths:
For injection: 20 mg melphalan flufenamide as a lyophilized powder in single‑dose vial reconstitution and dilution
Pepaxto isn’t a benefit for MSPERS and URMBT. |
| GROUP BENEFIT CHANGES |
Saginaw Plumbers and Pipe Fitters UA Local 85
|
Effective July 1, 2021, Saginaw Plumbers and Pipe Fitters UA Local 85, group number 71829, is moving platforms from MOS to Flexlink.
Group number: 71829
Alpha prefix: PPO (SYQ)
Platform: Flexlink
Plans offered:
PPO plans medical/surgical
Prescription drug plan |

Build COVID‑19 vaccine confidence with online resources from CDC, Health and Human Services
We’re getting closer to the State of Michigan’s goal to vaccinate 70% or more of Michigan’s population, but COVID‑19 vaccine hesitancy remains one of the major roadblocks. Despite the massive amount of information provided by national public health messengers, some residents are still on the fence about getting vaccinated or are refusing vaccination altogether.
Reasons for the hesitancy could be lack of trust in the public figures or perceiving the information relayed by them as conflicting. The most trusted messengers of COVID‑19 vaccine information are individual health care providers, according to the Kaiser Family Foundation’s COVID-19 Vaccine Monitor,** an ongoing research project launched in December 2020.
Online toolkits and resources
To help providers increase acceptance of the vaccine, the Centers for Disease Control and Prevention and the U.S. Department of Health and Human Services provide helpful online toolkits for providers to address vaccine hesitancy not only among patients, but also among their staff.
Some of the resources in the CDC toolkits** include:
- Strategies for building confidence in medical center and clinic immunization coordinators
- Materials for communicating with health care providers
- Digital and print communication resources
- Printable stickers
- Samples of social media messages and graphics
The HHS COVID‑19 Public Education Campaign website** includes resources and toolkits, a vaccine hesitancy map, campaign ads and information about joining the COVID‑19 Community Corps, which emails weekly tips, news and resources to share with your community. One useful feature of the HHS website is that it allows you to target vaccine information using the filters below:
- Audience — by race or ethnicity, older adults, health care professionals, rural communities, etc.
- Format — social media, posters and flyers, video, informational content, etc.
- Language — Chinese, English, Filipino, Japanese, Korean, Spanish and Vietnamese
- Topic — building vaccine confidence, getting vaccinated, preventive measures, vaccine safety and efficacy, etc.
Remind your patients that they will have zero out-of-pocket costs or network requirements to receive any of the COVID‑19 vaccines. We support and appreciate your ongoing efforts in caring for our members and improving the health of the citizens in our community.
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Additional enhancements to TurningPoint musculoskeletal surgical quality and safety management program
In the June 2021 issue of The Record, we announced several enhancements to the TurningPoint Healthcare Solutions LLC musculoskeletal surgical quality and safety management program.
Since then, Blue Cross Blue Shield of Michigan, Blue Care Network and TurningPoint have identified several opportunities to enhance the pain management portion of the program. Changes include:
- Updating medical policies for several pain management procedures
- Updating questionnaires for pain management procedures
- Removing prior authorization requirements for select procedures
Changes to TurningPoint medical policies for some pain management procedures
As of May 25, 2021, TurningPoint updated pain management medical policies to align more closely with Centers for Medicare & Medicaid Services guidelines. Pain management medical policies have been updated as follows.
Frequency of sessions allowed for epidural steroid injections
| Previous policy |
Updated policy |
| Allowed a total of 3 ESI sessions in a 6-month period regardless of region (cervical/thoracic or lumbar) |
Allows 3 ESI sessions in a 6-month period per episode of pain per region (cervical/thoracic or lumbar) |
Number of levels allowed per session for epidural steroid injections
| Previous policy |
Updated policy |
| Allowed 1 ESI level regardless of type |
Number of levels allowed depends on type:
- 1 level is allowed for caudal, interlaminar or bilateral transforaminal
-
2 levels are allowed for unilateral transforaminal
|
Time frame requirements for conservative treatments
| Previous policies |
Updated policies |
| Required 6 weeks of conservative treatment for epidural steroid injections, facet joint injections and sacroiliac joint injections |
Require only 4 weeks of conservative treatment for epidural steroid injections, facet joint injections and sacroiliac joint injections |
Note: TurningPoint applies CMS local and national coverage determination guidelines for Medicare Plus Blue℠ and BCN Advantage℠ members. For more information about the criteria TurningPoint uses to make determinations on authorization requests for pain management procedures for all members, see this news item on the ereferrals.bcbsm.com website.
Pain management questionnaire updates
As of May 31, 2021, we stopped requiring answers to the following questions on the questionnaires within the TurningPoint Provider Portal and pain management fax forms:
- What is the patient’s current health status?
- Activities of daily living (ADL) functional status
- For a BMI over 30, which weight loss activities have been documented?
Soon, we’ll remove these questions from the questionnaires.
Removing prior authorization requirements for select procedures
For dates of service on or after July 1, 2021, we won’t require prior authorization for the following procedure codes for refilling and recharging pain pumps: *62367, *62368, *62369 and *62370.
Additional information
As a reminder, TurningPoint manages authorizations for orthopedic, pain management and spinal procedures for the following groups and members:
- Blue Cross commercial** — All fully insured groups, select self-funded groups and all members with individual coverage
- Medicare Plus Blue members
- BCN commercial members
- BCN Advantage members
**To determine whether you need to submit prior authorization requests for Blue Cross commercial members, see the document titled Determining whether Blue Cross commercial members require prior authorization for musculoskeletal surgeries and related procedures.
2021 InterQual® criteria to be implemented Aug. 2
Blue Cross Blue Shield of Michigan and Blue Care Network will start using 2021 InterQual criteria on Aug. 2, 2021, to make utilization management determinations.
Keep in mind that there are InterQual criteria for behavioral health services, as well as non-behavioral health services. However, for Blue Cross commercial members, New Directions, an independent company that manages behavioral health services for most Blue Cross members, uses its own criteria for making determinations on behavioral health authorization requests.
Additional information about behavioral health services is at the end of this article.
Non-behavioral health services
We’ll use updated criteria for all levels of care to make utilization management determinations for requests to authorize non-behavioral health services, subject to review, for the following members:
- Blue Cross commercial
- Medicare Plus Blue℠
- BCN commercial
- BCN Advantage℠
When clinical information is requested for a medical or surgical admission or for other services, we require submission of the specific components of the medical record that validate that the request meets the criteria.
Blue Cross and BCN also use local rules — modifications of InterQual criteria — in making utilization management determinations. The 2021 local rules will also go into effect on Aug. 2, 2021.
In early July, you’ll be able to access the updated versions of the modifications (local rules), as applicable, for:
- Blue Cross — on the Authorization Requirements & Criteria page in the Blue Cross section of our ereferrals.bcbsm.com website. You’ll see links to the criteria in both Blue Cross commercial and the Medicare Plus Blue sections of that page. You’ll also be able to find it in Provider Secured Services. After you log in, click on BCBSM Provider Publications and Resources. Click on Newsletters & Resources, click on Clinical Criteria & Resources and scroll down to the section titled BCBSM modifications to InterQual criteria.
- BCN — on the Authorization Requirements & Criteria page in the BCN section of our ereferrals.bcbsm.com website. Look under the Referral and authorization information heading.
Refer to the table below for more specific information on which criteria are used in making determinations for various types of non‑behavioral health authorization requests.
| Criteria |
Application |
| InterQual acute — Adult and pediatrics |
- Inpatient admissions
- Continued stay discharge readiness
|
| InterQual level of care — Subacute and skilled nursing facility |
- Subacute and skilled nursing facility admissions
- Continued stay discharge readiness
|
| InterQual rehabilitation — Adult and pediatrics |
- Inpatient admissions
- Continued stay and discharge readiness
|
| InterQual level of care — Long-term acute care |
- Long‑term acute care facility admissions
- Continued stay discharge readiness
|
| InterQual imaging |
- Imaging studies and X‑rays
|
| InterQual procedures — Adult and pediatrics |
- Surgery and invasive procedures
|
| Medicare coverage guidelines (as applicable) |
- Services that require clinical review for medical necessity and benefit determinations
|
| Blue Cross and BCN medical policies |
- Services that require clinical review for medical necessity
|
| BCN‑developed local rules (applies to BCN commercial and BCN Advantage) |
- Exceptions to the application of InterQual criteria that reflect BCN’s accepted practice standards
|
Note: The information in the table above applies to lines of business and members whose authorizations are managed by Blue Cross or BCN directly and not by an independent company that provides services to Blue Cross Blue Shield of Michigan.
Behavioral health services
On Aug. 2, 2021, we’ll begin using the 2021 InterQual® criteria to make utilization management determinations for behavioral health services for members with:
- Medicare Plus Blue
- BCN commercial
- BCN Advantage
In addition, certain types of determinations will be based on modifications to InterQual criteria or on local rules or medical policies, as shown in the table below.
| Products |
Modified InterQual criteria for: |
Local rules or medical policies for: |
| BCN commercial and BCN Advantage |
- Substance use disorders: partial hospital program and intensive outpatient program
- Mental health disorders: partial hospital program and intensive outpatient program
- Residential mental health treatment (adult, geriatric, child and adolescent members)
Note: Neither BCN commercial members with BCN1, BCN5 and BCN10 plans nor BCN Advantage members have residential mental health treatment benefits.
|
- Applied behavior analysis for autism spectrum disorder — for BCN commercial members only
-
Neurofeedback for attention deficit disorder and attention deficit hyperactivity disorder
-
Transcranial magnetic stimulation, or TMS
-
Telemedicine (telepsychiatry and teletherapy)
|
| Medicare Plus Blue |
- Substance use disorders: partial hospital program and intensive outpatient program
-
Mental health disorders: partial hospital program and intensive outpatient program
Note: Only State of Michigan Medicare Plus Blue members have intensive outpatient program benefits. |
- Telemedicine (telepsychiatry and teletherapy)
Note: Medicare Plus Blue members don’t have neurofeedback or TMS benefits. |
For more information on telemedicine, refer to the Blue Cross and BCN: Telehealth for behavioral health providers document.
In early July, we’ll have links to the updated versions of the modified criteria, local rules and medical policies on these pages on our ereferrals.bcbsm.com website:
As noted earlier in the article, determinations on Blue Cross commercial behavioral health authorization requests are handled by New Directions. New Directions uses its own Medical Necessity Criteria.
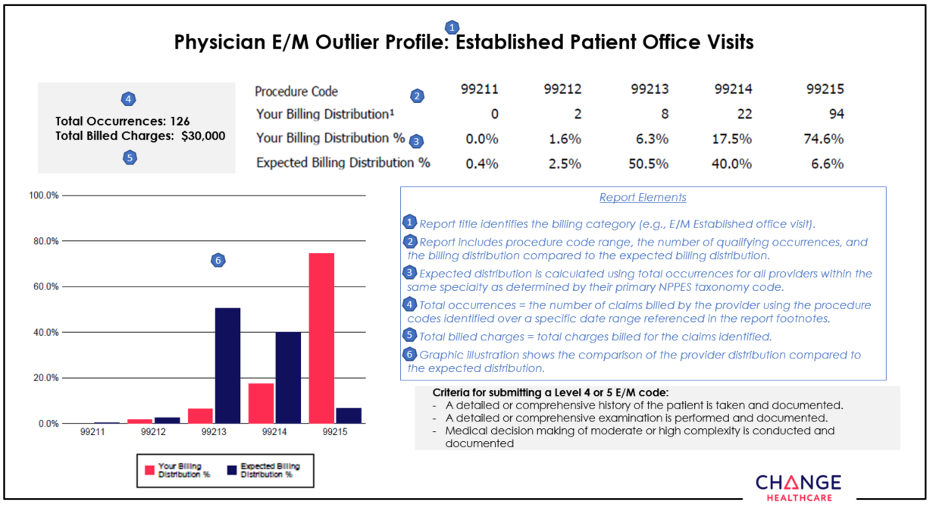
Change Healthcare continues outreach to educate providers about appropriate use of procedure codes
Selecting the CPT code that best reflects the complexity of an evaluation and management service can present a challenge for coders. Perhaps that’s why it’s not uncommon to make a mistake when selecting the appropriate E/M code.
To help health care providers and their office staff determine which CPT procedure code best reflects the complexity of a visit, Blue Cross Blue Shield of Michigan contracted with Change Healthcare, an independent company, to implement its Coding Advisor program in 2019.
Change Healthcare reviews E/M codes billed and other scenarios, such as modifier 25, observation care and nursing facility care, on claims submitted to Blue Cross. The program provides useful data insights to the provider community and works to maximize coding efficiency and accuracy through up-front education, rather than a traditional post-claim review process.
In July, Change Healthcare will reach out by phone or letter to providers who submit claims to Blue Cross with E/M codes. Coding Advisor will compare the billing of those codes to the codes used by their peers through a physician profile. See an example of a physician profile at the end of this article.
Throughout the course of this program, Coding Advisor will continue to monitor billing practices and send updated reports periodically. The company may contact your practice to discuss coding variances and to offer one-on-one coding education. All correspondence will be sent to you from Change Healthcare.
If you have any questions, call Change Healthcare Customer Support at 1‑844‑592‑7009, Option 3.
ClaimsXten to include additional professional and outpatient facility edits, starting in October
Starting in October 2021, ClaimsXten™ will edit additional services for professional and outpatient facility providers on Blue Cross Blue Shield of Michigan commercial claims. These new edits are part of our ongoing effort to promote correct coding and enhance our claims payment systems.
If you receive one of these edits, submit a corrected claim when appropriate. If you believe the services rendered warrant an exception, submit a clinical editing appeal with medical records.
Here are highlights of what these rules identify and edit:
- Identifies global obstetric care codes (defined as containing antepartum, delivery and postpartum services) and evaluates history to determine if another global OB care code or a component code, such as antepartum care, postpartum care or delivery‑only services has been submitted (professional claims only)
- Claims containing code pairs found to be unbundled, according to Centers for Medicare & Medicaid Services Integrated Outpatient Code Editor (outpatient facility claims only)
- Procedures that don’t warrant multiple submissions of a procedure or group of procedure codes on a single date of service, or across a date‑of‑service range, when billed by the same or different providers (professional claims only)
- Claim lines where the CMS medically unlikely edits, or MUE, has been exceeded for a CPT or HCPCS code, when reported by the same provider, for the same member, on the same date of service (outpatient facility claims only)
- Claim lines where the CMS MUE has been exceeded for a CPT or HCPCS code, when reported by the same provider, for the same member, on the same date of service (professional claims only)
- Claim lines with procedure codes that are eligible for the CMS Multiple Procedure Payment Reduction on the Technical Component of Diagnostic Ophthalmology (professional claims only)
- Claim lines that are eligible for pay percent adjustments for multiple quantity. Also applies pay percent adjustments when an eligible procedure code is billed with the modifier 78, FY or FX (professional claims only)
- Multiple endoscopy procedures, reported within the same family, and application of the multiple endoscopy pay percent reduction per CMS guidelines. This applies to surgeon and assistant surgeon (professional claims only).
- Home health care claims that are billed in excess of the allowed number of visits per day (facility claims only).
- Pre‑admission testing when the services are rendered the same day as an inpatient DRG admission or up to seven days prior to an inpatient DRG admission (facility claims only)
- Trauma activation on facility claims if an ambulance transport isn’t paid in history (facility claims only)
When appropriate, Blue Cross will support the use of modifiers that indicate unique circumstances for individual patients. The use of modifiers should be documented in the patient’s medical records.
Reminder: Modifier 59
What you need to know
You must submit medical records when using modifier 59 on four or more claims lines.
Remember to submit medical records when using modifier 59 on four or more claims lines. If modifier 59 is appended to more than four services, the claim will suspend to allow manual review of lines appended with modifier 59 when attachments are included; otherwise, each service appended with modifier 59 will be denied for lack of supporting documentation.
As modifier 59 is indicative of a distinct and separate service, more than four procedures reported with modifier 59 are examined for the additional services provided.
If you receive a denial related to this policy and disagree with the payment determination, don’t resubmit your claim or use the Medical Record Routing Form process. Instead, follow the Clinical Editing appeals process outlined below and include medical records.
- Appeals must be submitted with a Clinical Editing Appeal form. All required fields must be completed or the appeal will be returned to you. This form is available on the Blue Cross Blue Shield of Michigan provider site and in the provider manual on web‑DENIS.
- Submit the form one of the following ways:
Fax: 1‑866‑392‑7191
Mail: Clinical Editing Appeals
Mail Code G820
Blue Cross Blue Shield of Michigan PPO
611 Cascade West Parkway, SE
Grand Rapids, MI 49546-2143
Appeals must be submitted within 180 days of the original clinical editing denial. Documentation supporting the appeal must be submitted with the appeal.
Documentation requirements may vary depending on the service being appealed. As examples:
- Office services that have denied may require office notes.
- Services denied as duplicates will require records for both the denied and paid service to show that more than one was performed.
- Surgical denials may require operative reports.
It’s important to look at the denial reason and submit documentation appropriate to the procedure code that was denied and the reason for denial.
None of the information included herein is intended to be legal advice and as such it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
Refer our members only to network DME suppliers
What you need to know
When obtaining durable medical equipment for Blue Cross Blue Shield of Michigan and Blue Care Network members, health care providers are contractually obligated to obtain equipment from network suppliers. Otherwise, members may be responsible from the costs that arise from using an out‑of‑network supplier.
When obtaining durable medical equipment, or DME, for our members, you must use suppliers who are part of the Blue Cross Blue Shield of Michigan or Blue Care Network supplier network. Your contract with us obligates you to do this. The only exceptions are for emergencies or for other situations described in the policies we publish.
Here are two guidelines to keep in mind:
- Don’t refer to DME suppliers who are outside of our network.
- You must determine whether a particular DME supplier participates with the member’s plan before referring a member to the supplier.
How to identify a network DME supplier
For certain Blue Cross commercial members, you can find a network DME supplier by using the Find a Doctor tool on bcbsm.com. This applies only to Blue Cross commercial members who either:
- Have coverage through the Michigan Public School Employees' Retirement System, or
- Are Ford or General Motors salaried employees
For other members, use a supplier that’s part of the Northwood, Inc., network. This applies to:
- Blue Cross commercial members who don’t have coverage through one of the groups referred to above
- Medicare Plus Blue℠ members
- BCN commercial members
- BCN Advantage℠ members
To identify a supplier in the Northwood network, call Northwood at 1‑800‑393‑6432.
What happens when you use an out‑of‑network DME supplier
When you use out‑of‑network DME suppliers, members may be responsible for additional out‑of‑pocket costs. They may also be subject to balance billing by the suppliers because the suppliers aren’t in the Blue Cross or BCN network or aren’t following medical necessity requirements for replenishing supplies.
Our goal
Our goal is to partner with you to ensure that our members have convenient access to appropriate high‑quality, cost‑effective DME supplies that meet their clinical needs and that are covered by the plan they have.
Updated questionnaire available in e‑referral system
What you need to know
When we make utilization management determinations on your authorization requests, keep in mind that we use:
- Our authorization criteria
- Our medical policies
- Your answers to the questionnaires in the e‑referral system
On May 9, 2021, we updated the Sacral nerve neuromodulation/stimulation questionnaire in the e‑referral system.
We also updated the corresponding preview questionnaire on the ereferrals.bcbsm.com website. It shows the questions that appear in the e-referral system, so you can prepare your answers ahead of time.
To find the preview questionnaire:
- Click on Authorization Requirements & Criteria.
- In the section titled For Medicare Plus Blue PPO members, look under the label titled Authorization criteria and preview questionnaires — Medicare Plus Blue PPO.
The pertinent authorization criteria and medical policies are also available on the Authorization Requirements & Criteria page.
Submit prior authorization requests for CAR‑T cell therapy drugs administered to Medicare Advantage members in inpatient setting through NovoLogix
Before administering CAR‑T cell therapy drugs for Medicare Plus Blue℠ or BCN Advantage℠ members in an inpatient setting, you must complete these steps:
- Submit the request for the CAR‑T cell therapy drug, including all relevant clinical documentation in one of the following ways:
- Through the NovoLogix® online tool. (See the “NovoLogix” section below for more information.)
- By sending a fax to the Pharmacy Part B help desk at 1‑866‑392‑6465.
- Submit a separate request for the inpatient admission and other inpatient services (not including the CAR‑T cell therapy drug) through the e-referral system, as usual.
For the inpatient admission, follow the steps in the “Submit an inpatient authorization” section of the e‑referral User Guide.
If you have questions, email us at MASRX@bcbsm.com.
As a reminder:
- CAR‑T cell therapy drugs are covered under the medical benefit. Examples of CAR‑T cell therapy drugs are Yescarta®, Kymriah®, Tecartus™, Breyanzi® and Abecma®.
- Submit requests for outpatient administration of CAR‑T cell drugs through NovoLogix. There is no change to how outpatient requests are submitted.
- Prior authorization for CAR‑T drugs is not managed by AIM Specialty Health®.
NovoLogix
NovoLogix offers real‑time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services at bcbsm.com, you already have access to enter authorization requests through NovoLogix.
If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form, and fax it to the number on the form.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
Use correct HCPCS code for Spravato
What you need to know
Use HCPCS code S0013 for Spravato for dates of service on or after Jan. 1, 2021.
The Centers for Medicare & Medicaid Services established the permanent HCPCS code of S0013 for Spravato®, a medical benefit drug, to be used for dates of service on or after Jan. 1, 2021. However, many health care providers are using the older codes for these newer dates of service. This has resulted in problems with reimbursing claims.
We first communicated about this in the article titled “HCPCS replacement codes established,” in the March 2021 issue of The Record.
Prior authorization information
Providers must request prior authorization for Spravato when it’s administered in outpatient settings for:
- Members covered through Blue Cross Blue Shield of Michigan commercial fully insured groups except for groups that have opted out of the prior authorization program. Note: For groups that have opted out of the prior authorization program, Spravato is covered for the FDA‑approved indications.
- Blue Cross commercial members with individual coverage
- Medicare Plus Blue℠ members
- Blue Care Network commercial members
- BCN Advantage℠ members
Additional information
For more information on requirements related to drugs covered under the medical benefit, see the following documents:
Jemperli, Zynlonta require prior authorization for dates of service on or after July 26 for most members
For dates of service on or after July 26, 2021, we’re adding prior authorization requirements for the following drugs covered under the medical benefit:
- Jemperli™ (dostarlimab‑gxly), HCPCS codes J3490, J3590, J9999, C9399
- Zynlonta™ (loncastuximab tesirine‑lpyl), HCPCS codes J3490, J3590, J9999, C9399
Submit prior authorization requests through AIM Specialty Health®.
Prior authorization requirements apply when these drugs are administered in outpatient settings for:
- Blue Cross Blue Shield of Michigan commercial members who have coverage through fully insured groups and members with individual coverage
Exceptions: The Blue Cross commercial requirements don’t apply to members who have coverage through the Michigan Education Special Services Association or the Blue Cross and Blue Shield Federal Employee Program®, or to UAW Retiree Medical Benefits Trust non-Medicare members.
- Medicare Plus Blue℠
- Blue Care Network commercial
- BCN Advantage℠
How to submit authorization requests
Submit authorization requests to AIM using one of the following methods:
More about authorization requirements
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit, see:
- Blue Cross commercial and BCN commercial:
- Medicare Advantage:
We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Additional drugs to require prior authorization for Blue Cross’ URMBT non‑Medicare members
For dates of service on or after Sept. 7, 2021, four more drugs will require prior authorization when administered in an outpatient setting for Blue Cross Blue Shield of Michigan’s UAW Retiree Medical Benefits Trust non‑Medicare members.
Two of these drugs will also be subject to site‑of‑care requirements and waste avoidance quantity limits. All drugs listed in this article are covered under the medical benefit. Refer to the table below for more details.
| Drug |
Site of care |
Waste avoidance |
How to submit prior authorization requests |
Crysvita® (burosumab)
HCPCS code J0584 |
Yes |
No |
Submit prior‑authorization requests through the NovoLogix® online tool. It offers real‑time status checks and immediate approvals for certain medications.
To learn how to submit requests through NovoLogix, follow these steps:
- Go to ereferrals.bcbsm.com.
- Click on Blue Cross.
- Click on Medical Benefit Drugs.
- Scroll to the Blue Cross commercial column.
- Review How to submit requests electronically using NovoLogix
|
Luxturna®
(voretigene neparvovec)
HCPCS code J3398 |
No |
No |
Submit prior‑authorization requests through the NovoLogix® online tool. It offers real‑time status checks and immediate approvals for certain medications.
To learn how to submit requests through NovoLogix, follow these steps:
- Go to ereferrals.bcbsm.com.
- Click on Blue Cross.
- Click on Medical Benefit Drugs.
- Scroll to the Blue Cross commercial column.
- Review How to submit requests electronically using NovoLogix
|
Ultomiris® (ravulizumab)
HCPCS code J1303 |
Yes |
Yes |
Submit prior‑authorization requests through the NovoLogix® online tool. It offers real‑time status checks and immediate approvals for certain medications.
To learn how to submit requests through NovoLogix, follow these steps:
- Go to ereferrals.bcbsm.com.
- Click on Blue Cross.
- Click on Medical Benefit Drugs.
- Scroll to the Blue Cross commercial column.
- Review How to submit requests electronically using NovoLogix
|
Poteligeo® (mogamulizumab)
HCPCS code J9204 |
No |
No |
Submit prior‑authorization requests to AIM Specialty Health® using one of the following methods:
|
Explanation of other requirements
Through site‑of‑care requirements, members receiving select injectable or infusible drugs in the outpatient hospital setting are redirected to a lower cost, alternate site of care, such as the physician’s office or member’s home.
Through the waste avoidance program, dosing limits for these drugs will be based on weight and will be specific to:
- The dosing guidelines of the U.S. Food and Drug Administration and the manufacturer
- Current medical best practices
More about authorization requirements
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit, see:
Note: Accredo manages prior authorization requests for additional medical benefit drugs.
We’ll update our drug lists to reflect the information in this article prior to the effective date.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Lunch and learn webinars for physicians and coders focus on risk adjustment, coding
Action Item
Sign up now for live, monthly, lunchtime webinars.
We’re offering webinars that provide updated information on risk adjustment documentation and coding of common challenging diagnoses.
All sessions start at 12:15 p.m. Eastern time and run for 15 to 30 minutes. They also provide physicians and coders with an opportunity to ask questions.
Click on a link below to sign up for a webinar.
| Session date |
Topic |
Led by |
Sign-up link |
| Tuesday, July 20 |
Diabetes with complications |
Physician |
Register here
|
| Wednesday, Aug. 18 |
Renal disease |
Physician |
Register here
|
| Thursday, Sept. 23 |
Malignant neoplasm |
Physician |
Register here
|
| Tuesday, Oct. 12 |
Updates for ICD-10-CM |
Coder |
Register here |
| Wednesday, Nov. 17 |
Coding scenarios for primary care and specialty |
Coder |
Register here |
| Thursday, Dec. 9 |
E/M coding tips |
Coder |
Register here |
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding registration, email Patricia Scarlett at pscarlett@bcbsm.com.
None of the information included herein is intended to be legal advice and as such it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
New on‑demand training available
Action Item
Visit our new provider training site to find resources on topics that are important to your role.
Provider Experience continues to offer training resources for health care providers and staff, along with a new provider training site.
New resources
We’ve posted recordings of webinars previously delivered this year. In addition, video and eLearning modules are available on specific topics. The on-demand courses are designed to help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network.
Here’s a list of the newest resources:
- Evaluation and management guidelines for 2020 and beyond. This eLearning video reviews general coding and documentation requirements, evaluation and management guideline requirements and coding scenario examples.
- HCPCS and revenue code combinations for facility claims. This eLearning course teaches participants how to use the look‑up tools that help providers match HCPCS and CPT codes with the correct revenue codes for facility claims.
- Home Health Care Services Overview webinar. This webinar recording reviews the CareCentrix® home health care authorization program and provider portal.
- RBCE/MCG Self‑Service user videos. This series of videos demonstrates the steps for using the RCBE/MCG Self‑Service Tool to enroll new practitioners, modify status within your organization and make updates to practitioner affiliation.
New provider training site
Last month, we announced our new provider training site to enhance the training experience for health care providers and staff.
Active training courses and materials from 2019 through 2021 have moved from BCBSM Provider Training and BCN Learning Opportunities to the new training site. To request access, complete the following steps:
- Open the registration page.
- Complete the registration. We recommend using the same email you use to communicate with Blue Cross for provider‑related needs. This will become your login ID.
- Follow the link to the login page.
To learn more about the provider training site, watch this video that guides you through the experience. If you need assistance creating your login ID or navigating the site, contact ProviderTraining@bcbsm.com.
Reminder: Update your Provider Authorization form when changes occur
Blue Cross Blue Shield of Michigan is dedicated to safeguarding the protected health information of its members. These safeguards include completion of a Trading Partner Agreement and Provider Authorization form as part of the electronic data interchange setup process. All EDI trading partners must complete a TPA and Provider Authorization form before they can exchange PHI with Blue Cross.
The terms of the TPA require you to notify Blue Cross of any changes in your trading partner information, so it’s important to keep your Provider Authorization form up to date. Updating the form also ensures information is routed to the proper destination.
Update the form when you switch:
- Service bureaus or clearinghouses
- Software vendors
- Billing services
- 835-file recipients
- Submitter IDs
- Unique 835 receivers or Trading Partner IDs
Review the form when you:
- Join a new group practice.
- Leave a group practice and start billing using your own NPI.
- Hire a new billing service.
- Start submitting claims through a clearinghouse or when you’ve changed clearinghouses.
- Decide you no longer want to receive 835 remittance files.
- Select a new destination for your 835.
You don’t need to update the Provider Authorization form if your submitter and Trading Partner IDs don’t change.
How to change your EDI setup
To make changes to your EDI setup log in to the Trading Partner Agreement webpage. Or follow the complete navigation steps to arrive at the TPA login page:
- Visit bcbsm.com/providers
- Scroll down to the Provider resources section and click on Quick Links.
- Click on Electronic Connectivity EDI.
- Click on How to use EDI to exchange information with us electronically.
- Scroll down to the EDI agreements section and click on Update your Provider Authorization Form.
If you have any questions about EDI enrollment, contact the EDI Help Desk at EDISupport@bcbsm.com. Include your submitter ID with all correspondence.
Here are resources to help patients who are FEP members with diabetes and high blood pressure
This is part of an ongoing series of articles focusing on the tools and resources available to help FEP members manage their health.
As you know, diabetes and hypertension cause significant risks to public health. They play a major role in the development of cardiovascular disease and together account for almost two in three cases of chronic kidney disease, according to the American Diabetes Association.
The American Heart Association has joined forces with the American Diabetes Association to launch a program called Know Diabetes by Heart to reduce cardiovascular deaths, heart attacks and stroke. Five risk factors have been identified for patients to monitor:
- Body mass index and waist circumference
- Blood pressure
- A1c
- Cholesterol
- Kidney function
Monitoring the numbers associated with these risk factors can give patients a picture of their heart health and risk for heart disease and stroke. The Know Diabetes by Heart flyer, Diabetes & Heart Disease: The Numbers You Need to Know,** explains each risk factor and the target range for each risk factor, and provides a place for patients to record their numbers. More information can be found at knowdiabetesbyheart.org.**
The Blue Cross and Blue Shield Federal Employee Program® provides support programs to FEP members with chronic diseases, including diabetes and high blood pressure. The Care Management and wellness programs for Federal Employee Program members flyer describes programs and services offered to FEP members, along with contact information.
Members and health care providers can contact the FEP Customer Service at 1‑800‑482‑3600 if they have questions about benefits and wellness programs.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.

Additional enhancements to TurningPoint musculoskeletal surgical quality and safety management program
In the June 2021 issue of The Record, we announced several enhancements to the TurningPoint Healthcare Solutions LLC musculoskeletal surgical quality and safety management program.
Since then, Blue Cross Blue Shield of Michigan, Blue Care Network and TurningPoint have identified several opportunities to enhance the pain management portion of the program. Changes include:
- Updating medical policies for several pain management procedures
- Updating questionnaires for pain management procedures
- Removing prior authorization requirements for select procedures
Changes to TurningPoint medical policies for some pain management procedures
As of May 25, 2021, TurningPoint updated pain management medical policies to align more closely with Centers for Medicare & Medicaid Services guidelines. Pain management medical policies have been updated as follows.
Frequency of sessions allowed for epidural steroid injections
| Previous policy |
Updated policy |
| Allowed a total of 3 ESI sessions in a 6-month period regardless of region (cervical/thoracic or lumbar) |
Allows 3 ESI sessions in a 6-month period per episode of pain per region (cervical/thoracic or lumbar) |
Number of levels allowed per session for epidural steroid injections
| Previous policy |
Updated policy |
| Allowed 1 ESI level regardless of type |
Number of levels allowed depends on type:
- 1 level is allowed for caudal, interlaminar or bilateral transforaminal
-
2 levels are allowed for unilateral transforaminal
|
Time frame requirements for conservative treatments
| Previous policies |
Updated policies |
| Required 6 weeks of conservative treatment for epidural steroid injections, facet joint injections and sacroiliac joint injections |
Require only 4 weeks of conservative treatment for epidural steroid injections, facet joint injections and sacroiliac joint injections |
Note: TurningPoint applies CMS local and national coverage determination guidelines for Medicare Plus Blue℠ and BCN Advantage℠ members. For more information about the criteria TurningPoint uses to make determinations on authorization requests for pain management procedures for all members, see this news item on the ereferrals.bcbsm.com website.
Pain management questionnaire updates
As of May 31, 2021, we stopped requiring answers to the following questions on the questionnaires within the TurningPoint Provider Portal and pain management fax forms:
- What is the patient’s current health status?
- Activities of daily living (ADL) functional status
- For a BMI over 30, which weight loss activities have been documented?
Soon, we’ll remove these questions from the questionnaires.
Removing prior authorization requirements for select procedures
For dates of service on or after July 1, 2021, we won’t require prior authorization for the following procedure codes for refilling and recharging pain pumps: *62367, *62368, *62369 and *62370.
Additional information
As a reminder, TurningPoint manages authorizations for orthopedic, pain management and spinal procedures for the following groups and members:
- Blue Cross commercial** — All fully insured groups, select self-funded groups and all members with individual coverage
- Medicare Plus Blue members
- BCN commercial members
- BCN Advantage members
**To determine whether you need to submit prior authorization requests for Blue Cross commercial members, see the document titled Determining whether Blue Cross commercial members require prior authorization for musculoskeletal surgeries and related procedures.
2021 InterQual® criteria to be implemented Aug. 2
Blue Cross Blue Shield of Michigan and Blue Care Network will start using 2021 InterQual criteria on Aug. 2, 2021, to make utilization management determinations.
Keep in mind that there are InterQual criteria for behavioral health services, as well as non-behavioral health services. However, for Blue Cross commercial members, New Directions, an independent company that manages behavioral health services for most Blue Cross members, uses its own criteria for making determinations on behavioral health authorization requests.
Additional information about behavioral health services is at the end of this article.
Non-behavioral health services
We’ll use updated criteria for all levels of care to make utilization management determinations for requests to authorize non-behavioral health services, subject to review, for the following members:
- Blue Cross commercial
- Medicare Plus Blue℠
- BCN commercial
- BCN Advantage℠
When clinical information is requested for a medical or surgical admission or for other services, we require submission of the specific components of the medical record that validate that the request meets the criteria.
Blue Cross and BCN also use local rules — modifications of InterQual criteria — in making utilization management determinations. The 2021 local rules will also go into effect on Aug. 2, 2021.
In early July, you’ll be able to access the updated versions of the modifications (local rules), as applicable, for:
- Blue Cross — on the Authorization Requirements & Criteria page in the Blue Cross section of our ereferrals.bcbsm.com website. You’ll see links to the criteria in both Blue Cross commercial and the Medicare Plus Blue sections of that page. You’ll also be able to find it in Provider Secured Services. After you log in, click on BCBSM Provider Publications and Resources. Click on Newsletters & Resources, click on Clinical Criteria & Resources and scroll down to the section titled BCBSM modifications to InterQual criteria.
- BCN — on the Authorization Requirements & Criteria page in the BCN section of our ereferrals.bcbsm.com website. Look under the Referral and authorization information heading.
Refer to the table below for more specific information on which criteria are used in making determinations for various types of non‑behavioral health authorization requests.
| Criteria |
Application |
| InterQual acute — Adult and pediatrics |
- Inpatient admissions
- Continued stay discharge readiness
|
| InterQual level of care — Subacute and skilled nursing facility |
- Subacute and skilled nursing facility admissions
- Continued stay discharge readiness
|
| InterQual rehabilitation — Adult and pediatrics |
- Inpatient admissions
- Continued stay and discharge readiness
|
| InterQual level of care — Long-term acute care |
- Long‑term acute care facility admissions
- Continued stay discharge readiness
|
| InterQual imaging |
- Imaging studies and X‑rays
|
| InterQual procedures — Adult and pediatrics |
- Surgery and invasive procedures
|
| Medicare coverage guidelines (as applicable) |
- Services that require clinical review for medical necessity and benefit determinations
|
| Blue Cross and BCN medical policies |
- Services that require clinical review for medical necessity
|
| BCN‑developed local rules (applies to BCN commercial and BCN Advantage) |
- Exceptions to the application of InterQual criteria that reflect BCN’s accepted practice standards
|
Note: The information in the table above applies to lines of business and members whose authorizations are managed by Blue Cross or BCN directly and not by an independent company that provides services to Blue Cross Blue Shield of Michigan.
Behavioral health services
On Aug. 2, 2021, we’ll begin using the 2021 InterQual® criteria to make utilization management determinations for behavioral health services for members with:
- Medicare Plus Blue
- BCN commercial
- BCN Advantage
In addition, certain types of determinations will be based on modifications to InterQual criteria or on local rules or medical policies, as shown in the table below.
| Products |
Modified InterQual criteria for: |
Local rules or medical policies for: |
| BCN commercial and BCN Advantage |
- Substance use disorders: partial hospital program and intensive outpatient program
- Mental health disorders: partial hospital program and intensive outpatient program
- Residential mental health treatment (adult, geriatric, child and adolescent members)
Note: Neither BCN commercial members with BCN1, BCN5 and BCN10 plans nor BCN Advantage members have residential mental health treatment benefits.
|
- Applied behavior analysis for autism spectrum disorder — for BCN commercial members only
-
Neurofeedback for attention deficit disorder and attention deficit hyperactivity disorder
-
Transcranial magnetic stimulation, or TMS
-
Telemedicine (telepsychiatry and teletherapy)
|
| Medicare Plus Blue |
- Substance use disorders: partial hospital program and intensive outpatient program
-
Mental health disorders: partial hospital program and intensive outpatient program
Note: Only State of Michigan Medicare Plus Blue members have intensive outpatient program benefits. |
- Telemedicine (telepsychiatry and teletherapy)
Note: Medicare Plus Blue members don’t have neurofeedback or TMS benefits. |
For more information on telemedicine, refer to the Blue Cross and BCN: Telehealth for behavioral health providers document.
In early July, we’ll have links to the updated versions of the modified criteria, local rules and medical policies on these pages on our ereferrals.bcbsm.com website:
As noted earlier in the article, determinations on Blue Cross commercial behavioral health authorization requests are handled by New Directions. New Directions uses its own Medical Necessity Criteria.
ClaimsXten to include additional professional and outpatient facility edits, starting in October
Starting in October 2021, ClaimsXten™ will edit additional services for professional and outpatient facility providers on Blue Cross Blue Shield of Michigan commercial claims. These new edits are part of our ongoing effort to promote correct coding and enhance our claims payment systems.
If you receive one of these edits, submit a corrected claim when appropriate. If you believe the services rendered warrant an exception, submit a clinical editing appeal with medical records.
Here are highlights of what these rules identify and edit:
- Identifies global obstetric care codes (defined as containing antepartum, delivery and postpartum services) and evaluates history to determine if another global OB care code or a component code, such as antepartum care, postpartum care or delivery‑only services has been submitted (professional claims only)
- Claims containing code pairs found to be unbundled, according to Centers for Medicare & Medicaid Services Integrated Outpatient Code Editor (outpatient facility claims only)
- Procedures that don’t warrant multiple submissions of a procedure or group of procedure codes on a single date of service, or across a date‑of‑service range, when billed by the same or different providers (professional claims only)
- Claim lines where the CMS medically unlikely edits, or MUE, has been exceeded for a CPT or HCPCS code, when reported by the same provider, for the same member, on the same date of service (outpatient facility claims only)
- Claim lines where the CMS MUE has been exceeded for a CPT or HCPCS code, when reported by the same provider, for the same member, on the same date of service (professional claims only)
- Claim lines with procedure codes that are eligible for the CMS Multiple Procedure Payment Reduction on the Technical Component of Diagnostic Ophthalmology (professional claims only)
- Claim lines that are eligible for pay percent adjustments for multiple quantity. Also applies pay percent adjustments when an eligible procedure code is billed with the modifier 78, FY or FX (professional claims only)
- Multiple endoscopy procedures, reported within the same family, and application of the multiple endoscopy pay percent reduction per CMS guidelines. This applies to surgeon and assistant surgeon (professional claims only).
- Home health care claims that are billed in excess of the allowed number of visits per day (facility claims only).
- Pre‑admission testing when the services are rendered the same day as an inpatient DRG admission or up to seven days prior to an inpatient DRG admission (facility claims only)
- Trauma activation on facility claims if an ambulance transport isn’t paid in history (facility claims only)
When appropriate, Blue Cross will support the use of modifiers that indicate unique circumstances for individual patients. The use of modifiers should be documented in the patient’s medical records.
Submit prior authorization requests for CAR‑T cell therapy drugs administered to Medicare Advantage members in inpatient setting through NovoLogix
Before administering CAR‑T cell therapy drugs for Medicare Plus Blue℠ or BCN Advantage℠ members in an inpatient setting, you must complete these steps:
- Submit the request for the CAR‑T cell therapy drug, including all relevant clinical documentation in one of the following ways:
- Through the NovoLogix® online tool. (See the “NovoLogix” section below for more information.)
- By sending a fax to the Pharmacy Part B help desk at 1‑866‑392‑6465.
- Submit a separate request for the inpatient admission and other inpatient services (not including the CAR‑T cell therapy drug) through the e-referral system, as usual.
For the inpatient admission, follow the steps in the “Submit an inpatient authorization” section of the e‑referral User Guide.
If you have questions, email us at MASRX@bcbsm.com.
As a reminder:
- CAR‑T cell therapy drugs are covered under the medical benefit. Examples of CAR‑T cell therapy drugs are Yescarta®, Kymriah®, Tecartus™, Breyanzi® and Abecma®.
- Submit requests for outpatient administration of CAR‑T cell drugs through NovoLogix. There is no change to how outpatient requests are submitted.
- Prior authorization for CAR‑T drugs is not managed by AIM Specialty Health®.
NovoLogix
NovoLogix offers real‑time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services at bcbsm.com, you already have access to enter authorization requests through NovoLogix.
If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form, and fax it to the number on the form.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
Use correct HCPCS code for Spravato
What you need to know
Use HCPCS code S0013 for Spravato for dates of service on or after Jan. 1, 2021.
The Centers for Medicare & Medicaid Services established the permanent HCPCS code of S0013 for Spravato®, a medical benefit drug, to be used for dates of service on or after Jan. 1, 2021. However, many health care providers are using the older codes for these newer dates of service. This has resulted in problems with reimbursing claims.
We first communicated about this in the article titled “HCPCS replacement codes established,” in the March 2021 issue of The Record.
Prior authorization information
Providers must request prior authorization for Spravato when it’s administered in outpatient settings for:
- Members covered through Blue Cross Blue Shield of Michigan commercial fully insured groups except for groups that have opted out of the prior authorization program. Note: For groups that have opted out of the prior authorization program, Spravato is covered for the FDA‑approved indications.
- Blue Cross commercial members with individual coverage
- Medicare Plus Blue℠ members
- Blue Care Network commercial members
- BCN Advantage℠ members
Additional information
For more information on requirements related to drugs covered under the medical benefit, see the following documents:
Jemperli, Zynlonta require prior authorization for dates of service on or after July 26 for most members
For dates of service on or after July 26, 2021, we’re adding prior authorization requirements for the following drugs covered under the medical benefit:
- Jemperli™ (dostarlimab‑gxly), HCPCS codes J3490, J3590, J9999, C9399
- Zynlonta™ (loncastuximab tesirine‑lpyl), HCPCS codes J3490, J3590, J9999, C9399
Submit prior authorization requests through AIM Specialty Health®.
Prior authorization requirements apply when these drugs are administered in outpatient settings for:
- Blue Cross Blue Shield of Michigan commercial members who have coverage through fully insured groups and members with individual coverage
Exceptions: The Blue Cross commercial requirements don’t apply to members who have coverage through the Michigan Education Special Services Association or the Blue Cross and Blue Shield Federal Employee Program®, or to UAW Retiree Medical Benefits Trust non-Medicare members.
- Medicare Plus Blue℠
- Blue Care Network commercial
- BCN Advantage℠
How to submit authorization requests
Submit authorization requests to AIM using one of the following methods:
More about authorization requirements
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit, see:
- Blue Cross commercial and BCN commercial:
- Medicare Advantage:
We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Additional drugs to require prior authorization for Blue Cross’ URMBT non‑Medicare members
For dates of service on or after Sept. 7, 2021, four more drugs will require prior authorization when administered in an outpatient setting for Blue Cross Blue Shield of Michigan’s UAW Retiree Medical Benefits Trust non‑Medicare members.
Two of these drugs will also be subject to site‑of‑care requirements and waste avoidance quantity limits. All drugs listed in this article are covered under the medical benefit. Refer to the table below for more details.
| Drug |
Site of care |
Waste avoidance |
How to submit prior authorization requests |
Crysvita® (burosumab)
HCPCS code J0584 |
Yes |
No |
Submit prior‑authorization requests through the NovoLogix® online tool. It offers real‑time status checks and immediate approvals for certain medications.
To learn how to submit requests through NovoLogix, follow these steps:
- Go to ereferrals.bcbsm.com.
- Click on Blue Cross.
- Click on Medical Benefit Drugs.
- Scroll to the Blue Cross commercial column.
- Review How to submit requests electronically using NovoLogix
|
Luxturna®
(voretigene neparvovec)
HCPCS code J3398 |
No |
No |
Submit prior‑authorization requests through the NovoLogix® online tool. It offers real‑time status checks and immediate approvals for certain medications.
To learn how to submit requests through NovoLogix, follow these steps:
- Go to ereferrals.bcbsm.com.
- Click on Blue Cross.
- Click on Medical Benefit Drugs.
- Scroll to the Blue Cross commercial column.
- Review How to submit requests electronically using NovoLogix
|
Ultomiris® (ravulizumab)
HCPCS code J1303 |
Yes |
Yes |
Submit prior‑authorization requests through the NovoLogix® online tool. It offers real‑time status checks and immediate approvals for certain medications.
To learn how to submit requests through NovoLogix, follow these steps:
- Go to ereferrals.bcbsm.com.
- Click on Blue Cross.
- Click on Medical Benefit Drugs.
- Scroll to the Blue Cross commercial column.
- Review How to submit requests electronically using NovoLogix
|
Poteligeo® (mogamulizumab)
HCPCS code J9204 |
No |
No |
Submit prior‑authorization requests to AIM Specialty Health® using one of the following methods:
|
Explanation of other requirements
Through site‑of‑care requirements, members receiving select injectable or infusible drugs in the outpatient hospital setting are redirected to a lower cost, alternate site of care, such as the physician’s office or member’s home.
Through the waste avoidance program, dosing limits for these drugs will be based on weight and will be specific to:
- The dosing guidelines of the U.S. Food and Drug Administration and the manufacturer
- Current medical best practices
More about authorization requirements
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit, see:
Note: Accredo manages prior authorization requests for additional medical benefit drugs.
We’ll update our drug lists to reflect the information in this article prior to the effective date.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Lunch and learn webinars for physicians and coders focus on risk adjustment, coding
Action Item
Sign up now for live, monthly, lunchtime webinars.
We’re offering webinars that provide updated information on risk adjustment documentation and coding of common challenging diagnoses.
All sessions start at 12:15 p.m. Eastern time and run for 15 to 30 minutes. They also provide physicians and coders with an opportunity to ask questions.
Click on a link below to sign up for a webinar.
| Session date |
Topic |
Led by |
Sign-up link |
| Tuesday, July 20 |
Diabetes with complications |
Physician |
Register here
|
| Wednesday, Aug. 18 |
Renal disease |
Physician |
Register here
|
| Thursday, Sept. 23 |
Malignant neoplasm |
Physician |
Register here
|
| Tuesday, Oct. 12 |
Updates for ICD-10-CM |
Coder |
Register here |
| Wednesday, Nov. 17 |
Coding scenarios for primary care and specialty |
Coder |
Register here |
| Thursday, Dec. 9 |
E/M coding tips |
Coder |
Register here |
If you have any questions about the sessions, contact April Boyce at aboyce@bcbsm.com. If you have questions regarding registration, email Patricia Scarlett at pscarlett@bcbsm.com.
None of the information included herein is intended to be legal advice and as such it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
New on‑demand training available
Action Item
Visit our new provider training site to find resources on topics that are important to your role.
Provider Experience continues to offer training resources for health care providers and staff, along with a new provider training site.
New resources
We’ve posted recordings of webinars previously delivered this year. In addition, video and eLearning modules are available on specific topics. The on-demand courses are designed to help you work more efficiently with Blue Cross Blue Shield of Michigan and Blue Care Network.
Here’s a list of the newest resources:
- Evaluation and management guidelines for 2020 and beyond. This eLearning video reviews general coding and documentation requirements, evaluation and management guideline requirements and coding scenario examples.
- HCPCS and revenue code combinations for facility claims. This eLearning course teaches participants how to use the look‑up tools that help providers match HCPCS and CPT codes with the correct revenue codes for facility claims.
- Home Health Care Services Overview webinar. This webinar recording reviews the CareCentrix® home health care authorization program and provider portal.
- RBCE/MCG Self‑Service user videos. This series of videos demonstrates the steps for using the RCBE/MCG Self‑Service Tool to enroll new practitioners, modify status within your organization and make updates to practitioner affiliation.
New provider training site
Last month, we announced our new provider training site to enhance the training experience for health care providers and staff.
Active training courses and materials from 2019 through 2021 have moved from BCBSM Provider Training and BCN Learning Opportunities to the new training site. To request access, complete the following steps:
- Open the registration page.
- Complete the registration. We recommend using the same email you use to communicate with Blue Cross for provider‑related needs. This will become your login ID.
- Follow the link to the login page.
To learn more about the provider training site, watch this video that guides you through the experience. If you need assistance creating your login ID or navigating the site, contact ProviderTraining@bcbsm.com.
Reminder: Update your Provider Authorization form when changes occur
Blue Cross Blue Shield of Michigan is dedicated to safeguarding the protected health information of its members. These safeguards include completion of a Trading Partner Agreement and Provider Authorization form as part of the electronic data interchange setup process. All EDI trading partners must complete a TPA and Provider Authorization form before they can exchange PHI with Blue Cross.
The terms of the TPA require you to notify Blue Cross of any changes in your trading partner information, so it’s important to keep your Provider Authorization form up to date. Updating the form also ensures information is routed to the proper destination.
Update the form when you switch:
- Service bureaus or clearinghouses
- Software vendors
- Billing services
- 835-file recipients
- Submitter IDs
- Unique 835 receivers or Trading Partner IDs
Review the form when you:
- Join a new group practice.
- Leave a group practice and start billing using your own NPI.
- Hire a new billing service.
- Start submitting claims through a clearinghouse or when you’ve changed clearinghouses.
- Decide you no longer want to receive 835 remittance files.
- Select a new destination for your 835.
You don’t need to update the Provider Authorization form if your submitter and Trading Partner IDs don’t change.
How to change your EDI setup
To make changes to your EDI setup log in to the Trading Partner Agreement webpage. Or follow the complete navigation steps to arrive at the TPA login page:
- Visit bcbsm.com/providers
- Scroll down to the Provider resources section and click on Quick Links.
- Click on Electronic Connectivity EDI.
- Click on How to use EDI to exchange information with us electronically.
- Scroll down to the EDI agreements section and click on Update your Provider Authorization Form.
If you have any questions about EDI enrollment, contact the EDI Help Desk at EDISupport@bcbsm.com. Include your submitter ID with all correspondence.

Submit prior authorization requests for CAR‑T cell therapy drugs administered to Medicare Advantage members in inpatient setting through NovoLogix
Before administering CAR‑T cell therapy drugs for Medicare Plus Blue℠ or BCN Advantage℠ members in an inpatient setting, you must complete these steps:
- Submit the request for the CAR‑T cell therapy drug, including all relevant clinical documentation in one of the following ways:
- Through the NovoLogix® online tool. (See the “NovoLogix” section below for more information.)
- By sending a fax to the Pharmacy Part B help desk at 1‑866‑392‑6465.
- Submit a separate request for the inpatient admission and other inpatient services (not including the CAR‑T cell therapy drug) through the e-referral system, as usual.
For the inpatient admission, follow the steps in the “Submit an inpatient authorization” section of the e‑referral User Guide.
If you have questions, email us at MASRX@bcbsm.com.
As a reminder:
- CAR‑T cell therapy drugs are covered under the medical benefit. Examples of CAR‑T cell therapy drugs are Yescarta®, Kymriah®, Tecartus™, Breyanzi® and Abecma®.
- Submit requests for outpatient administration of CAR‑T cell drugs through NovoLogix. There is no change to how outpatient requests are submitted.
- Prior authorization for CAR‑T drugs is not managed by AIM Specialty Health®.
NovoLogix
NovoLogix offers real‑time status checks and immediate approvals for certain medications. If you have access to Provider Secured Services at bcbsm.com, you already have access to enter authorization requests through NovoLogix.
If you need to request access to Provider Secured Services, complete the Provider Secured Access Application form, and fax it to the number on the form.
List of requirements
For a list of requirements related to drugs covered under the medical benefit, see the Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Blue PPO and BCN Advantage members.
Use correct HCPCS code for Spravato
What you need to know
Use HCPCS code S0013 for Spravato for dates of service on or after Jan. 1, 2021.
The Centers for Medicare & Medicaid Services established the permanent HCPCS code of S0013 for Spravato®, a medical benefit drug, to be used for dates of service on or after Jan. 1, 2021. However, many health care providers are using the older codes for these newer dates of service. This has resulted in problems with reimbursing claims.
We first communicated about this in the article titled “HCPCS replacement codes established,” in the March 2021 issue of The Record.
Prior authorization information
Providers must request prior authorization for Spravato when it’s administered in outpatient settings for:
- Members covered through Blue Cross Blue Shield of Michigan commercial fully insured groups except for groups that have opted out of the prior authorization program. Note: For groups that have opted out of the prior authorization program, Spravato is covered for the FDA‑approved indications.
- Blue Cross commercial members with individual coverage
- Medicare Plus Blue℠ members
- Blue Care Network commercial members
- BCN Advantage℠ members
Additional information
For more information on requirements related to drugs covered under the medical benefit, see the following documents:
Jemperli, Zynlonta require prior authorization for dates of service on or after July 26 for most members
For dates of service on or after July 26, 2021, we’re adding prior authorization requirements for the following drugs covered under the medical benefit:
- Jemperli™ (dostarlimab‑gxly), HCPCS codes J3490, J3590, J9999, C9399
- Zynlonta™ (loncastuximab tesirine‑lpyl), HCPCS codes J3490, J3590, J9999, C9399
Submit prior authorization requests through AIM Specialty Health®.
Prior authorization requirements apply when these drugs are administered in outpatient settings for:
- Blue Cross Blue Shield of Michigan commercial members who have coverage through fully insured groups and members with individual coverage
Exceptions: The Blue Cross commercial requirements don’t apply to members who have coverage through the Michigan Education Special Services Association or the Blue Cross and Blue Shield Federal Employee Program®, or to UAW Retiree Medical Benefits Trust non-Medicare members.
- Medicare Plus Blue℠
- Blue Care Network commercial
- BCN Advantage℠
How to submit authorization requests
Submit authorization requests to AIM using one of the following methods:
More about authorization requirements
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit, see:
- Blue Cross commercial and BCN commercial:
- Medicare Advantage:
We’ll update the appropriate drug lists to reflect the information in this message prior to the effective date.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Additional drugs to require prior authorization for Blue Cross’ URMBT non‑Medicare members
For dates of service on or after Sept. 7, 2021, four more drugs will require prior authorization when administered in an outpatient setting for Blue Cross Blue Shield of Michigan’s UAW Retiree Medical Benefits Trust non‑Medicare members.
Two of these drugs will also be subject to site‑of‑care requirements and waste avoidance quantity limits. All drugs listed in this article are covered under the medical benefit. Refer to the table below for more details.
| Drug |
Site of care |
Waste avoidance |
How to submit prior authorization requests |
Crysvita® (burosumab)
HCPCS code J0584 |
Yes |
No |
Submit prior‑authorization requests through the NovoLogix® online tool. It offers real‑time status checks and immediate approvals for certain medications.
To learn how to submit requests through NovoLogix, follow these steps:
- Go to ereferrals.bcbsm.com.
- Click on Blue Cross.
- Click on Medical Benefit Drugs.
- Scroll to the Blue Cross commercial column.
- Review How to submit requests electronically using NovoLogix
|
Luxturna®
(voretigene neparvovec)
HCPCS code J3398 |
No |
No |
Submit prior‑authorization requests through the NovoLogix® online tool. It offers real‑time status checks and immediate approvals for certain medications.
To learn how to submit requests through NovoLogix, follow these steps:
- Go to ereferrals.bcbsm.com.
- Click on Blue Cross.
- Click on Medical Benefit Drugs.
- Scroll to the Blue Cross commercial column.
- Review How to submit requests electronically using NovoLogix
|
Ultomiris® (ravulizumab)
HCPCS code J1303 |
Yes |
Yes |
Submit prior‑authorization requests through the NovoLogix® online tool. It offers real‑time status checks and immediate approvals for certain medications.
To learn how to submit requests through NovoLogix, follow these steps:
- Go to ereferrals.bcbsm.com.
- Click on Blue Cross.
- Click on Medical Benefit Drugs.
- Scroll to the Blue Cross commercial column.
- Review How to submit requests electronically using NovoLogix
|
Poteligeo® (mogamulizumab)
HCPCS code J9204 |
No |
No |
Submit prior‑authorization requests to AIM Specialty Health® using one of the following methods:
|
Explanation of other requirements
Through site‑of‑care requirements, members receiving select injectable or infusible drugs in the outpatient hospital setting are redirected to a lower cost, alternate site of care, such as the physician’s office or member’s home.
Through the waste avoidance program, dosing limits for these drugs will be based on weight and will be specific to:
- The dosing guidelines of the U.S. Food and Drug Administration and the manufacturer
- Current medical best practices
More about authorization requirements
Authorization isn’t a guarantee of payment. As always, health care practitioners need to verify eligibility and benefits for members.
For additional information on requirements related to drugs covered under the medical benefit, see:
Note: Accredo manages prior authorization requests for additional medical benefit drugs.
We’ll update our drug lists to reflect the information in this article prior to the effective date.
**Blue Cross Blue Shield of Michigan doesn’t own or control this website.

Refer our members only to network DME suppliers
What you need to know
When obtaining durable medical equipment for Blue Cross Blue Shield of Michigan and Blue Care Network members, health care providers are contractually obligated to obtain equipment from network suppliers. Otherwise, members may be responsible from the costs that arise from using an out‑of‑network supplier.
When obtaining durable medical equipment, or DME, for our members, you must use suppliers who are part of the Blue Cross Blue Shield of Michigan or Blue Care Network supplier network. Your contract with us obligates you to do this. The only exceptions are for emergencies or for other situations described in the policies we publish.
Here are two guidelines to keep in mind:
- Don’t refer to DME suppliers who are outside of our network.
- You must determine whether a particular DME supplier participates with the member’s plan before referring a member to the supplier.
How to identify a network DME supplier
For certain Blue Cross commercial members, you can find a network DME supplier by using the Find a Doctor tool on bcbsm.com. This applies only to Blue Cross commercial members who either:
- Have coverage through the Michigan Public School Employees' Retirement System, or
- Are Ford or General Motors salaried employees
For other members, use a supplier that’s part of the Northwood, Inc., network. This applies to:
- Blue Cross commercial members who don’t have coverage through one of the groups referred to above
- Medicare Plus Blue℠ members
- BCN commercial members
- BCN Advantage℠ members
To identify a supplier in the Northwood network, call Northwood at 1‑800‑393‑6432.
What happens when you use an out‑of‑network DME supplier
When you use out‑of‑network DME suppliers, members may be responsible for additional out‑of‑pocket costs. They may also be subject to balance billing by the suppliers because the suppliers aren’t in the Blue Cross or BCN network or aren’t following medical necessity requirements for replenishing supplies.
Our goal
Our goal is to partner with you to ensure that our members have convenient access to appropriate high‑quality, cost‑effective DME supplies that meet their clinical needs and that are covered by the plan they have.
| 
